Page Updated:
Jul 2021
The arrythmia monitoring devices market in Europe is expected to grow from US$ 1,739.79 million in 2021 to US$ 2,638.61 million by 2028; it is estimated to grow at a CAGR of 6.1% from 2021 to 2028.
The UK, Germany, and France are major economies in Europe. Growing geriatric population base is the major factor driving the growth of the Europe arrythmia monitoring devices market. The elderly population in the world is anticipated to reach 1.4 billion in 2030, 2.1 billion in 2050, and more than 3.1 billion in 2100. According to the Global Ageing 2019 survey, the world's population of people with age 65 and above totaled 703 million in 2019. The elderly population is expected to double to 1.5 billion by 2050. The frequency of people with age 65 or above is likely to increase from 1 in 11 to 1 in 6 from 2019 to 2025. According to the WHO, the percentage of people with age 60 and above is estimated to reach 22% by 2050 from 12% in 2015. In 2017, the European Union had ~25% of people with age 60 and above. By 2050, all regions of the world, except Africa, would have ~25% or more of their inhabitants with age 60 and above. Although arrhythmias are common among people of all age groups, their incidence is rising significantly among elderly people. As per the report "Older Americans 2016: Key Indicators of Well-Being" by the Federal Interagency Forum on Aging-Related Statistics, 35.8% of individuals with age 85 and above had a mild or extreme cognitive disorder. Therefore, the rise in prevalence of various diseases among geriatric populations is adding to the demand for arrhythmia monitoring devices, which is further anticipated to drive the market in Europe.The European economy is severely affected due to the exponential growth of COVID-19 cases in the region. Spain, Italy, Germany, France, and the UK are among the most affected European countries. As per the Worldometer, as of 23 June 2021, Spain, UK, Italy, Germany, and France recorded 4,651,988; 4,254,294; 3,731,287; and 5,760,002, COVID-19 cases respectively, and the deaths toll is also high in these countries. The current coronavirus (COVID-19) outbreak has been declared by WHO as a public health emergency of international concern according to the International Health Regulation. Also, the pandemic has halted the adoption of new medical device policy and delayed clinical trials and disrupted processes. Two consecutive waves of coronavirus had devastating impact on economic activities in the Europe. Implementation of social distancing and lockdown policies led to closing of cardiac centers and patient visits for elective heart treatments. This factor negatively impacted the adoption of arrhythmia monitoring devices in the region. The European Commission recently postponed the application date of the MDR for one-year due to COVID-19 pandemic. The Medical Device Regulation (MDR) requires manufacturers to conduct Post Market Clinical Follow-Up (PMCF) studies to demonstrate the continued safety and performance of their devices. This is expected to impact the new product launches and ongoing clinical trials of medical devices, impacting sales and growth of the Europe arrhythmia monitoring devices market. In addition, disrupted supply chains, extended lockdowns, and cancelling of travel visits of sales personnel of companies have also negativity affected the growth of the arrhythmia monitoring devices market 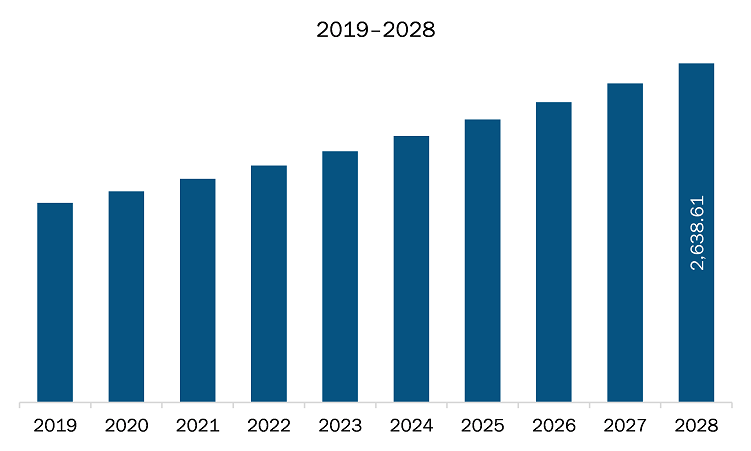
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Europe Arrythmia Monitoring Devices Market Segmentation
Europe Arrythmia Monitoring Devices Market – By Type
- ECG Monitors
- Implantable Monitors
- Holter Monitors
- Mobile Cardiac Telemetry
- Others
Europe Arrythmia Monitoring Devices Market – By Application
- Bradycardia
- Tachycardia
- Atrial Fibrillation
- Ventricular Fibrillation
- Premature Contraction
- Others
Europe Arrythmia Monitoring Devices Market – By End User
- Hospitals and Clinics
- Ambulatory Centers
- Diagnostic Centers
- Others
Europe Arrythmia Monitoring Devices Market, by Country
- U.K.
- Germany
- France
- Italy
- Spain
- Rest of Europe
Europe Arrythmia Monitoring Devices Market -Companies Mentioned
- Abbott
- AiveCor, Inc
- Biotronik, Inc.
- General Electric Company
- Hill-Rom Holding Inc.
- Koninklijke Philips N.V.
- Medicalgorithmics
- Medtronic
- OSI Systems, Inc
Europe Arrythmia Monitoring Devices Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 1,739.79 Million |
| Market Size by 2028 | US$ 2,638.61 Million |
| CAGR (2021 - 2028) | 6.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Our Clients






























Sales Assistance
US: +1-646-491-9876
UK: +44-20-8125-4005
Email:
sales@theinsightpartners.com
Chat with us

87-673-9708

ISO 9001:2015





 Get Free Sample For
Get Free Sample For