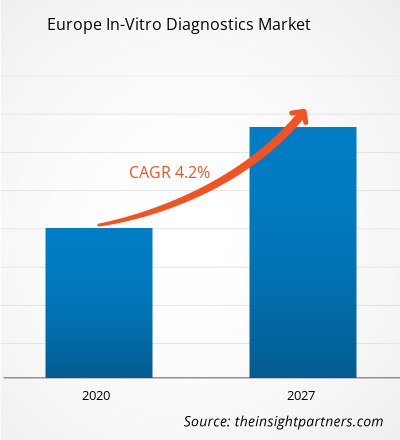
The In-vitro diagnostics market in Europe is expected to grow from US$ 15,973.0 million in 2019 to US$ 22,221.8 million by 2027; it is estimated to grow at a CAGR of 4.2% from 2020 to 2027.
The region is witnessing exponential increase in prevalence of infectious diseases. These diseases are prominently caused due to pathogenic microorganisms, such as viruses, bacteria, and parasites. Certain factors, such as poor sanitary conditions, lack of public hygiene, massive air pollution, and lack of safe drinking water, are playing a significant role in the increasing prevalence of infectious diseases. For instance, according to data published by the World Health Organization (WHO) in 2019, approximately 71.0 million people suffered from hepatitis C virus infection across the globe. Moreover, extreme globalization, intense mobility of the population, and persistent urbanization are expected to spread viral infections with greater ease. Since there is a lack of better public health surveillance systems and access to care, a substantial population in developing countries as well as remote regions, is highly prone to viral infections. This, combined with considerable growth in the viral epidemics, is projected to offer a high demand the in-vitro diagnostics (IVD) during the forecast period
The coronavirus pandemic has offered unprecedented growth opportunities and challenges for the European region's in-vitro diagnostic market. The in-vitro diagnostics market has experienced profitable growth due to the raised awareness and increased demand for in-vitro diagnostics tests. The IVD product manufacturers primarily supported the growth of the market. The manufacturers were at the frontline and have invested in the expertise, production, distribution of the IVD products. On the other hand, there are several regulatory challenges concerning IVD. The regulatory bodies are involved in the development of new in vitro Diagnostic Regulation (IVDR). It is expected that the IVDR will be fully implemented from May 26, 2022. According to the new regulations, manufacturers will have to re-certify all tests on time and the infrastructure facilities. In contrast, there have been significant installations of PCR and qPCR in the laboratories and hospitals to improve the capacity of COVID-19 tests. Countries such as Italy, Spain, and the UK have faced a significant number of patients. Therefore, these countries have significantly contributed to the growth of the in-vitro diagnostics market
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the In-vitro diagnostics market. The Europe In-vitro diagnostics market is expected to grow at a good CAGR during the forecast period.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Europe In-Vitro Diagnostics Market Segmentation
By Product and Services
- Instruments
- Reagents & Kits
- Software & Services
By Technology
- Immunoassay/ Immunochemistry
- Clinical Chemistry
- Molecular Diagnostics
- Microbiology
- Blood Glucose Self-Monitoring
- Coagulation & Hemostasis
- Hematology
- Urinalysis
- Others
By Application
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Autoimmune Diseases
- Nephrology
- Others
By End User
- Hospitals
- Laboratories
- Homecare
- Others
By Country
Europe
- Germany
- UK
- France
- Spain
- Italy
- Rest of Europe
Companies Mentioned
- F. HOFFMANN-LA ROCHE LTD.
- DANAHER
- ABBOTT
- SIEMENS AG
- SYSMEX CORPORATION
- THERMO FISHER SCIENTIFIC, INC.
- BD
- BIOMERIEUX SA
- BIO-RAD LABORATORIES, INC.
- QIAGEN
Europe In-Vitro Diagnostics Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 15,973.0 Million |
| Market Size by 2027 | US$ 22,221.8 Million |
| Global CAGR (2020 - 2027) | 4.2% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Product and Services
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product and Services, Technology, Application, End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
UK, Germany, France, Italy, Russia
Trends and growth analysis reports related to Life Sciences : READ MORE..
- F. HOFFMANN-LA ROCHE LTD.
- DANAHER
- ABBOTT
- SIEMENS AG
- SYSMEX CORPORATION
- THERMO FISHER SCIENTIFIC, INC.
- BD
- BIOMERIEUX SA
- BIO-RAD LABORATORIES, INC.
- QIAGEN
 Get Free Sample For
Get Free Sample For