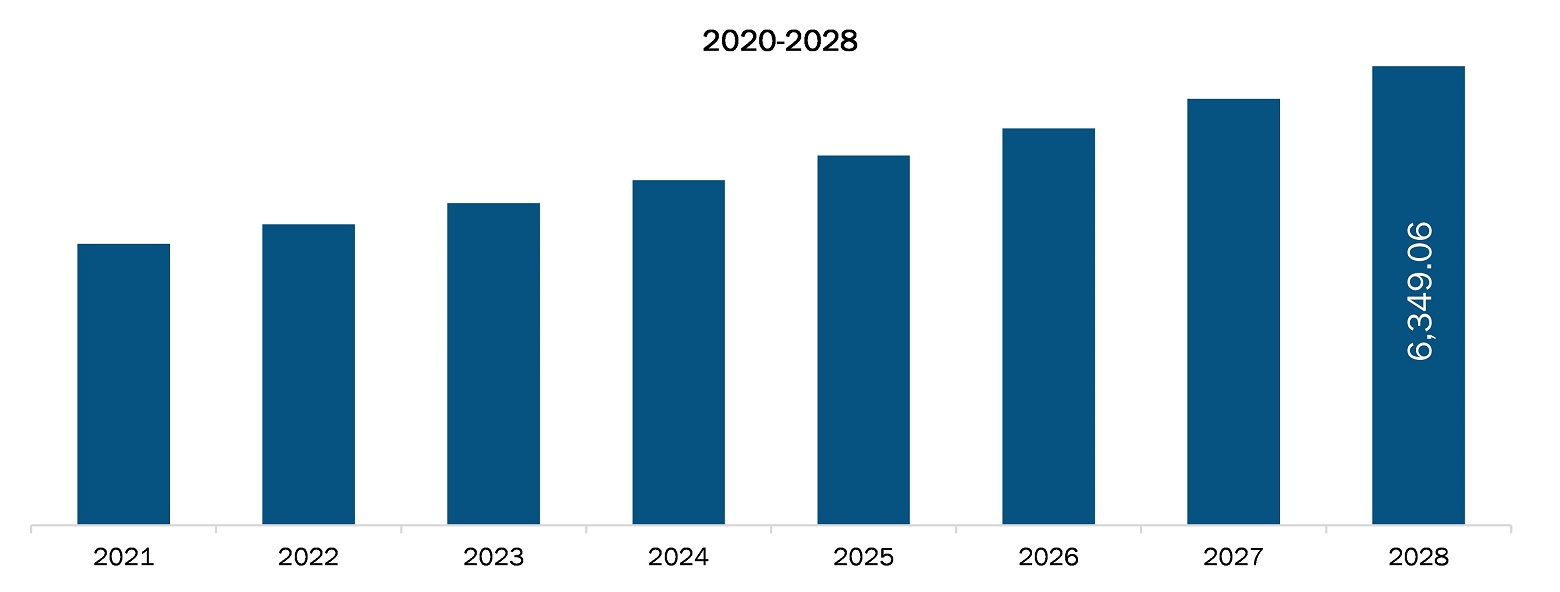
The European laboratory developed test market is projected to reach US$ 8,988.78 million by 2028 from US$ 5,664.43 million in 2021; it is estimated to grow at a CAGR of 6.8% from 2021 to 2028.
The increasing incidence of cancer and genetic disorders and the growing number of product launches across Europe are factors propelling the laboratory developed testing market. However, the changing regulatory landscape is hampering the growth of the laboratory development test market.LDTs are developed and used within laboratories and not distributed or sold to other laboratories or healthcare facilities. These tests are designed to overcome the challenge of the unavailability of commercial tests. Many LDTs are genetic tests developed for rare diseases. Thus, the frequency of development and introduction of new LDTs is high. In November 2020, Aditx Therapeutics, Inc received a CLIA certification for AditxtScore laboratory operations for immune monitoring, which allowed them to launch the AditxtScore test to diagnose COVID-19. The increasing emergence of SARS-CoV-2 variants has highlighted the need to identify, trace, and track mutations across the complete viral genome. In January 2021, Eurofins launched the NovaType test for detecting and monitoring new variants of SARS-CoV-2. This RT-PCR assay is clinically validated for identifying B.1.1.7 and B.1.351 variants with a short time. NovaType is already available as an LDT in Germany, and it will be made available in over 50 Eurofins laboratories worldwide for testing patients for COVID-19. Also, the rising demand for personalized medicine is offering significant growth opportunities for the players operating in the European laboratory developed test market.
Countries across Europe are witnessing a resurgence in COVID-19 cases after successfully mitigating the pandemic. Many countries are declaring more cases each day than the first wave in 2020. The governments of many countries are working toward scaling up testing capacity, which is further driving the laboratory developed test market. Many market players have launched products to meet the growing demand. For instance, in August 2020, Eurofins Technologies (Luxembourg) launched a range of testing kits for serology-based antibody detection by ELISA of patients exposed to COVID-19. These tests are designed to meet the region-specific requirements for COVID-19. Viracor Eurofins received Food and Drug Administration (FDA) and Emergency Use Authorization (EUA) for its SARS-CoV-2 laboratory developed test. Other Eurofins laboratories have developed alternative RT-PCR options to meet local regulatory obligations and mitigate reagent supply chain issues globally. However, other non-emergency tests witnessed a drop due to lockdown measures and overburdened healthcare institutions.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
EUROPE LABORATORY DEVELOPED TEST MARKET SEGMENTATION
By Type
- Clinical Biochemistry
- Critical Care
- Haematology
-
- Coagulation and Hemostasis,
- Hemoglobin Testing
- Blood Count Testing
- Others
- Immunology
- Microbiology
- Molecular Diagnostics
- Other Test Types
By Application
- Academic Institutes
- Clinical Research organizations
- Hospitals laboratory
- Specialty Diagnostic Centers
- Others
By Country
- Italy
- France
- Germany
- Spain
- UK
- Rest of Europe
Company Profiles
- Quest Diagnostics Incorporated
- F.HOFFMANN-LA ROCHE LTD
- QIAGEN
- Illumina, Inc.
- Eurofins Scientific
- Guardant Health
Europe Laboratory Developed Test Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 5,664.43 Million |
| Market Size by 2028 | US$ 8,988.78 Million |
| CAGR (2021 - 2028) | 6.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For