The pediatric medical devices market in Europe is expected to grow from US$ 8,253.86 million in 2021 to US$ 13,862.68 million by 2028; it is estimated to register a CAGR of 7.7% from 2021 to 2028.
France, Germany, Italy, Spain, and UK are major economies in Europe. Pediatric medical devices available in wide rage is the major factor driving the growth of the Europe pediatric medical devices market. Pediatric care is comparatively different from that for adult patients. The medical devices used are based on essential technologies and principles. In the Europe pediatric medical devices markets larger number of medical devices manufacturers offer a more comprehensive range of devices and services related to the products. The international players have established a strong network of products sales and post-sales services. The Europe market leaders such as Koninklijke Philips N.V., MEDTRONIC, Siemens AG, GENERAL ELECTRIC, and other companies associated with medical devices related business have set business units, manufacturing plants and sales & services offices in international locations. The presence of such business units facilitates the customers and the patients in the regions, thereby garnering brand loyalty. The Europe players are also adopting online platforms to sell their products to hospitals and directly to the patients. Thus, doorstep services help in better customer care and support in acquiring market in remote locations due to advanced logistics systems and extended distributions network of the companies and associated partners. Moreover, the rising awareness about at-home medical devices and the adoption of remote patient care services are expected to support the market in the forecast period.
The European market is severely hit due to the exponential increase in COVID-19 cases in the region. Many nations are now reporting more patients per day than during the first wave earlier this year. Lockdowns are being reintroduced in the UK, Spain, and Italy, and Ireland. Due to the second wave of COVID-19 crisis, the governments of many countries are heading toward mounting up testing capacity. The European countries were profoundly affected due to the COVID-19 pandemic. Countries such as Italy, Spain, and France have recorded the most significant number of positive cases and have registered the maximum number of deaths.The rising rate of COVID-19 cases has increased stress on the region's healthcare system, thereby propelling the demand for diagnostic tests in its healthcare system and supporting the expansion of the sector in this region. Moreover, regulatory bodies in the region are taking preventive measures such as shutting down business operations. In March 2020, the European Medicines Agency established a managing committee to deal with the impact of COVID-19 on the supply chain of medicines. Many studies revealed the clinical features of COVID-19 in adults and infants. Limited knowledge about characteristics, results, and intrauterine transmission potential in pediatric aged 28 days or less have chances to acquire COVID-19. Due to the fear of infection in babies, people are refraining from hospitals, gynecology clinics, and baby care centers for neonatal care. These measures are likely to hamper the overall manufacturing and marketing of pediatric medical devices, which will have an adverse impact on market growth.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the Europe pediatric medical devices market. The Europe pediatric medical devices market is expected to grow at a good CAGR during the forecast period.
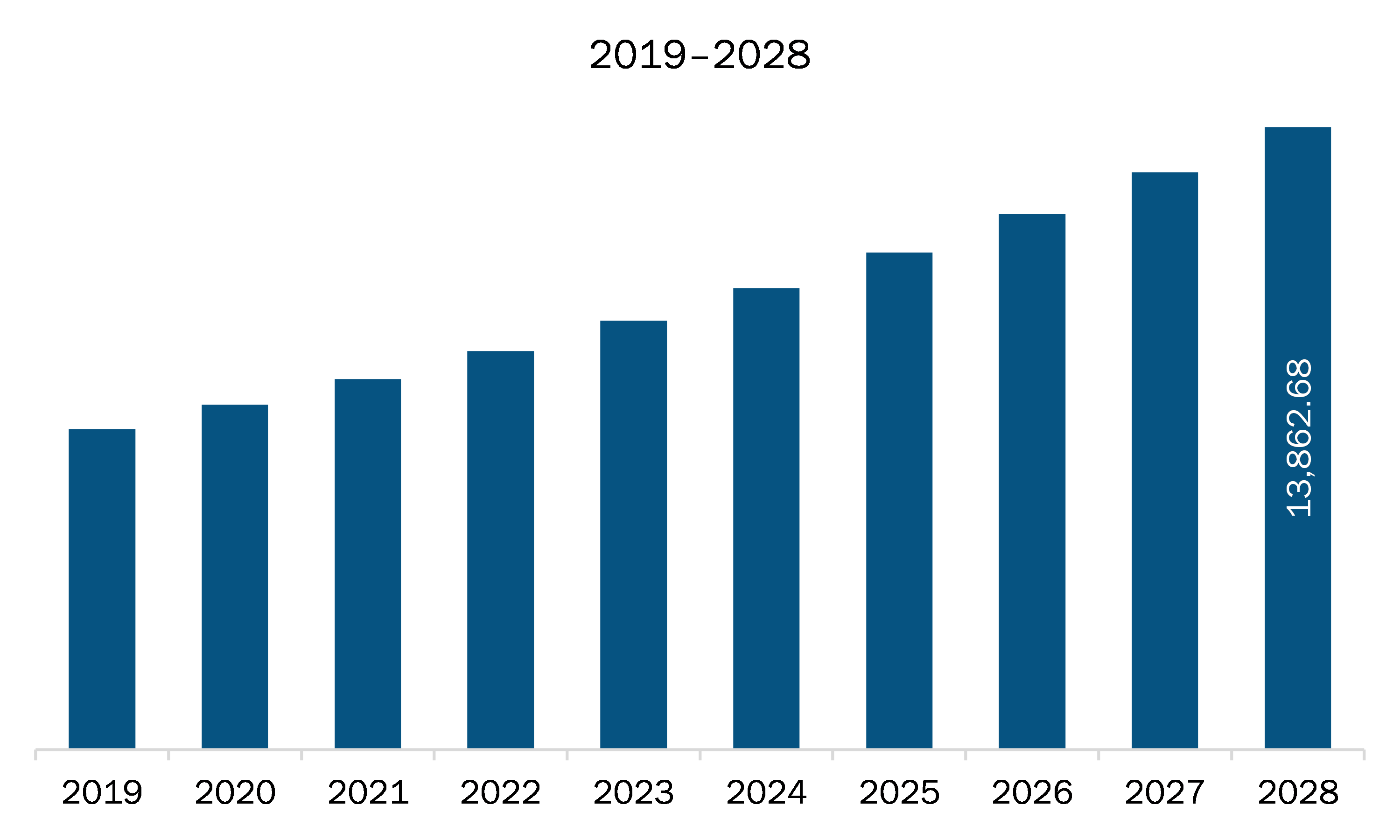
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Europe Pediatric Medical Devices Market Segmentation
Europe Pediatric Medical Devices Market – By Product
- In Vitro Diagnostic (IVD) Devices
- Cardiology Devices
- Respiratory Care Devices
- Monitoring Devices
- Neonatal ICU Devices
- Others
Europe Pediatric Medical Devices Market – By End User
- Hospitals
- Pediatric Clinics
- Others
Europe Pediatric Medical Devices Market, by Country
- France
- Germany
- Italy
- Spain
- UK
- Rest of Europe
Europe Pediatric Medical Devices Market - Companies Mentioned
- Atom Medical Corp.
- F. Hoffmann-La Roche Ltd.
- Fritz Stephan GmbH
- General Electric Company
- Hamilton Medical
- Koninklijke Philips N.V.
- Medtronic
- Siemens AG
- TSE MEDICAL
Europe Pediatric Medical Devices Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 8,253.86 Million |
| Market Size by 2028 | US$ 13,862.68 Million |
| CAGR (2021 - 2028) | 7.7% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For