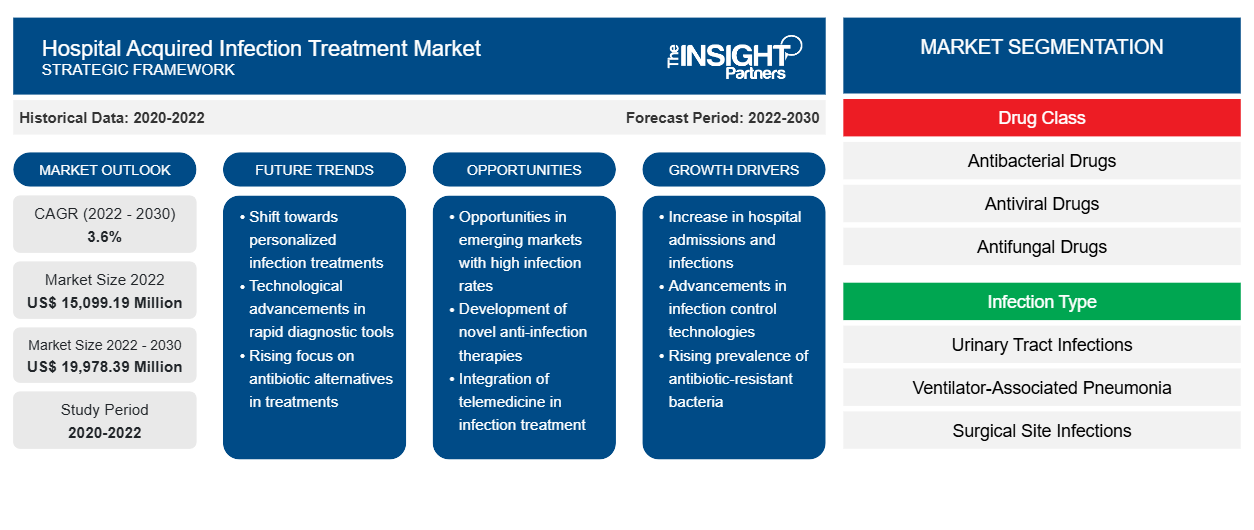
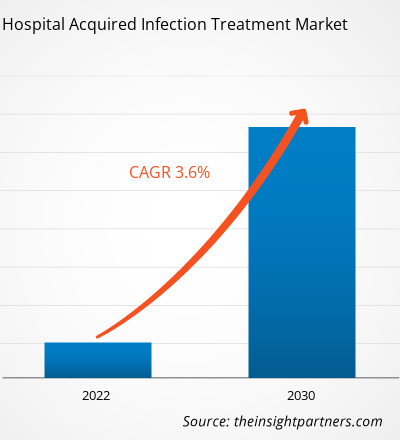
[Research Report] The hospital acquired infection treatment market is expected to grow from US$ 15,099.19 million in 2022 to US$ 19,978.39 million by 2030; it is anticipated to record a CAGR of 3.6% from 2022 to 2030.
Market Insights and Analyst View:
Hospital-acquired infections (HAIs), also known as nosocomially acquired infections, may develop in a variety of settings, including hospitals, long-term care institutions, and ambulatory settings, and they can also appear after discharge. HAIs can also involve occupational infections that affect healthcare workers. Key factors driving the hospital acquired infection treatment market growth are high prevalence of HAIs and growing focus on patient safety and quality care. However, the threat of antimicrobial resistance hinders hospital acquired infection treatment market growth.
Growth Drivers and Restraints:
Central line-associated bloodstream infections, catheter-associated urinary tract infections, and ventilator-associated pneumonia are a few examples of healthcare-associated infections (HAIs). These infections, known as surgical site infections, can also arise at surgical sites. According to the Centers for Disease Control and Prevention (CDC) report “2020 National and State Healthcare-Associated Infections Progress Report" published in 2021, cases of central line-associated bloodstream infections, Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia, and ventilator-associated events increased by 24%, 35%, and 15%, respectively, in the US between 2019 and 2020.
Furthermore, According to the January 2022 report from the National Healthcare Safety Network (NHSN), urinary tract infections (UTIs) are the fifth most common type of healthcare-associated infection. UTIs account for more than 9.5% of acute care hospital conditions. Catheter-Associated Urinary Tract Infection (CAUTI) is caused by urinary catheters used to drain urine from the bladder. CAUTI is one of the most frequent infections among hospitalized UTIs patients. According to the Centers for Disease Control and Prevention (CDC), nearly 75% of infections are related to a urinary catheter. During their hospital stays, 15% to 25% of hospitalized patients need to use urinary catheters. Prolonged urinary catheter use is the most major risk factor for getting CAUTI. CAUTI complications entail patient suffering, prolonged hospital stays, increased treatment costs, and even death.
Furthermore, according to the CDC, UTIs are responsible for more than 13,000 deaths each year. Acute uncomplicated cystitis causes around six days of discomfort, resulting in ∼7 million office visits every year at a cost of US$ 1.6 billion. As a result, rising number of HAI cases places a significant financial burden on the healthcare system. The increasing prevalence of CAUTI, combined with the increased demand for drugs for treatment, is propelling the market.
However, antimicrobial resistance poses a significant challenge to HAI treatment, limiting the efficacy of existing antimicrobial agents and necessitating the development of novel treatment strategies and antimicrobial alternatives to combat resistant pathogens effectively.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Hospital Acquired Infection Treatment Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Hospital Acquired Infection Treatment Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The global hospital acquired infection treatment market is segmented on the basis of drug class, infection type, and distribution channel. Based on drug class, the hospital acquired infection treatment market is segmented into antibacterial drugs, antiviral drugs, antifungal drugs, and others. Based on infection type, the hospital acquired infection treatment is differentiated into urinary tract infections, ventilator-associated pneumonia, surgical site infections, bloodstream infections, and other hospital infections. Based on distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, e-commerce, and others. The hospital acquired infection treatment market, based on geography, is segmented into North America (the US, Canada, and Mexico), Europe (Germany, France, Italy, the UK, Russia, and the Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and the Rest of Asia Pacific), the Middle East & Africa (South Africa, Saudi Arabia, the UAE, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Segmental Analysis:
The hospital acquired infection treatment market, by drug class, is segmented into antibacterial drugs, antiviral drugs, antifungal drugs, and others. The antibacterial drugs segment held the largest market share in 2022 and is anticipated to register the highest CAGR during 2022–2030.
The hospital acquired infection treatment market, by infection type, is segmented into urinary tract infections, ventilator-associated pneumonia, surgical site infections, bloodstream infections, and other hospital infections. The urinary tract infections segment held the largest market share in 2022. However, the ventilator-associated pneumonia segment is anticipated to register the highest CAGR from 2022 to 2030.
The hospital acquired infection treatment market, by distribution channel, is segmented into hospital pharmacies, retail pharmacies, e-commerce, and others. In 2022, the hospital pharmacies segment held the largest market share, and the same segment is anticipated to register the highest CAGR during 2022–2030.
Regional Analysis:
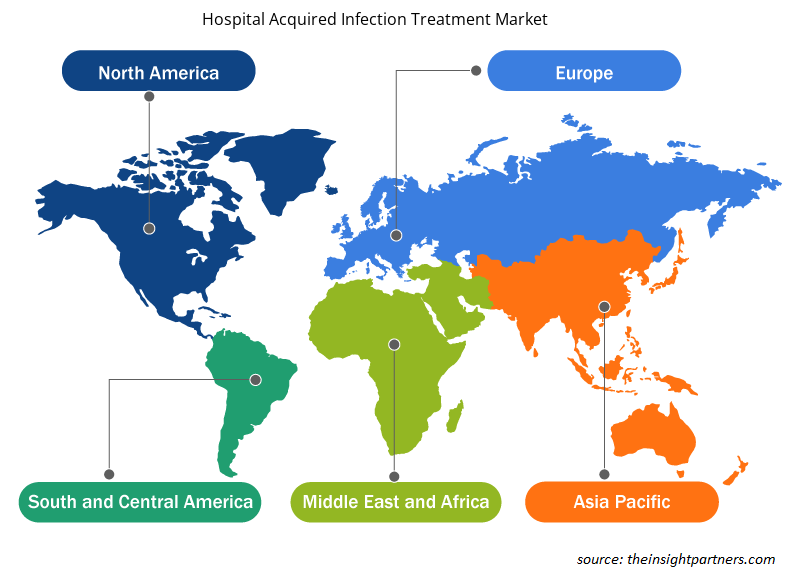
Based on geography, the global hospital acquired infection treatment market is segmented into five key regions—North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa.
In 2022, North America held the largest share of the global hospital acquired infection treatment market size. The US has a high prevalence of HAI, which propels the hospital acquired infection treatment market growth. As per the CDC, on any given day, about 1 in 31 hospital patients has at least one HAI. Similarly, according to the Office of Disease Prevention and Health Promotion, HAIs and other infections can lead to sepsis, and it causes an ∼1.7 million illness cases and 270,000 deaths per year in the US. Thus, the high prevalence of HAI among people in the US fuels the hospital acquired infection treatment market growth.
Industry Developments and Future Opportunities:
Various initiatives taken by key players operating in the global hospital acquired infection treatment market are listed below:
- In May 2023, Innoviva Specialty Therapeutics announced that the US Food and Drug Administration (FDA) had approved XACDURO (sulbactam for injection; durlobactam for injection), co-packaged for intravenous use in patients aged 18 years and above for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex (Acinetobacter). Innoviva Specialty Therapeutics stated that it is focused on delivering innovative therapies in critical care and infectious diseases.
- In May 2023, Researchers of the Indian Institute of Science Education and Research (IISER), Pune and Central Drug Research Institute (CSIR-CDRI), Lucknow announced that they had discovered a potential new antibiotic against Acinetobacter baumannii, which frequently causes HAIs.
- In September 2020, Shionogi & Co Ltd announced that the FDA had approved a supplemental New Drug Application (sNDA) for FETROJA (cefiderocol) for the treatment of patients aged 18 years and above with hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible Gram-negative microorganisms such as Enterobacter cloacae complex, Klebsiella pneumoniae, Acinetobacter baumannii complex, Escherichia coli, Pseudomonas aeruginosa, and Serratia marcescens.
- In June 2020, Merck announced that the US Food and Drug Administration (FDA) had approved a supplemental New Drug Application (sNDA) for RECARBRIO (imipenem, cilastatin, and relebactam) for the treatment of patients aged 18 years and above suffering from hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP), caused by susceptible Gram-negative microorganisms such as Acinetobacter calcoaceticus-baumannii complex, Klebsiella oxytoca, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella aerogenes, Pseudomonas aeruginosa, and Serratia marcescens.
Hospital Acquired Infection Treatment Market Regional Insights
The regional trends and factors influencing the Hospital Acquired Infection Treatment Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Hospital Acquired Infection Treatment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Hospital Acquired Infection Treatment Market
Hospital Acquired Infection Treatment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 15,099.19 Million |
| Market Size by 2030 | US$ 19,978.39 Million |
| Global CAGR (2022 - 2030) | 3.6% |
| Historical Data | 2020-2022 |
| Forecast period | 2022-2030 |
| Segments Covered |
By Drug Class
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Hospital Acquired Infection Treatment Market Players Density: Understanding Its Impact on Business Dynamics
The Hospital Acquired Infection Treatment Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Hospital Acquired Infection Treatment Market are:
- Merck & Co Inc
- Pfizer Inc
- AbbVie Inc
- Paratek Pharmaceuticals Inc
- Eugia US LLC
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Hospital Acquired Infection Treatment Market top key players overview
Competitive Landscape and Key Companies:
Merck & Co Inc, Pfizer Inc, AbbVie Inc, Paratek Pharmaceuticals Inc, Eugia US LLC, Abbott Laboratories, Cumberland Pharmaceuticals Inc, Innoviva Specialty Therapeutics Inc, Cipla Ltd, and Eli Lilly and Company are among the prominent players operating in the hospital acquired infection treatment market. These companies focus on new technologies, advancements in existing products, and geographic expansions to meet the growing consumer demand worldwide and increase their product range in specialty portfolios.
Frequently Asked Questions
What is hospital acquired infection treatment?
Hospital-acquired infections (HAI), also known as nosocomially acquired infections. HAIs are infections acquired during medical treatment that were not present at the time of admission. They may develop in a variety of settings, including hospitals, long-term care institutions, and ambulatory settings, and they can also appear after discharge. HAIs can also involve occupational infections that affect healthcare workers.
Who are the major players in the hospital acquired infection treatment market?
The hospital acquired infection treatment market majorly consists of the players, including Merck & Co Inc, Pfizer Inc, AbbVie Inc, Paratek Pharmaceuticals Inc, Eugia US LLC, Abbott Laboratories, Cumberland Pharmaceuticals Inc, Innoviva Specialty Therapeutics Inc, Cipla Ltd, and Eli Lilly and Company.
What factors drive the hospital acquired infection treatment market?
Key factors driving the hospital acquired infection treatment market growth include the high prevalence of hospital-acquired infections (HAI) and the growing focus on patient safety and quality care.
What was the estimated hospital acquired infection treatment market size in 2022?
The hospital acquired infection treatment market was valued at US$ 15,099.19 million in 2022.
Which distribution channel segment dominates the hospital acquired infection treatment market?
The hospital acquired infection treatment market, by distribution channel, is segmented into hospital pharmacies, retail pharmacies, e-commerce, and others. In 2022, the hospitals pharmacies segment held the largest market share, and the same segment is anticipated to register the highest CAGR during 2022–2030.
What are the growth estimates for the hospital acquired infection treatment market till 2030?
The hospital acquired infection treatment market is expected to be valued at US$ 19,978.39 million in 2030.
Which infection type segment dominates the hospital acquired infection treatment market?
The hospital acquired infection treatment market, by infection type, is segmented into urinary tract infections, ventilator-associated pneumonia, surgical site infections, bloodstream infections, and other hospital infections. The urinary tract infections segment held a larger market share in 2022; However, the ventilator-associated pneumonia segment is anticipated to register a higher CAGR during 2022-2030.
Which drug class segment dominates the hospital acquired infection treatment market?
The hospital acquired infection treatment market, by drug class, is segmented into antibacterial drugs, antiviral drugs, antifungal drugs, and others. The antibacterial drugs segment held a larger market share in 2022, and the same segment is anticipated to register a higher CAGR during 2022-2030.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
I wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA, MANAGING DIRECTOR, PineCrest Healthcare Ltd.The Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
Yukihiko Adachi CEO, Deep Blue, LLC.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Strategic Planning
- Investment Justification
- Identifying Emerging Markets
- Enhancing Marketing Strategies
- Boosting Operational Efficiency
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely — analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You’ll receive access to the report within 4–6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we’ll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we’re happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you’re considering, and we’ll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report’s scope, methodology, customization options, or which license suits you best, we’re here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we’ll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we’ll ensure the issue is resolved quickly so you can access your report without interruption.















The List of Companies - Hospital Acquired Infection Treatment Market
- Merck & Co Inc
- Pfizer Inc
- AbbVie Inc
- Paratek Pharmaceuticals Inc
- Eugia US LLC
- Abbott Laboratories
- Cumberland Pharmaceuticals Inc
- Innoviva Specialty Therapeutics Inc
- Cipla Ltd
- Eli Lilly and Company






 Get Free Sample For
Get Free Sample For