Laboratory Developed Test Market Size Trends & Key Opportunities 2034
Laboratory Developed Test Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Clinical Biochemistry, Critical Care, Hematology, Microbiology, Molecular Diagnostics, Immunology, and Others) and Application (Academic Institutes, Clinical Research Organizations, Hospitals Laboratory, Specialty Diagnostic Centers, and Others)
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Apr 2026
- Report Code : TIPRE00019603
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
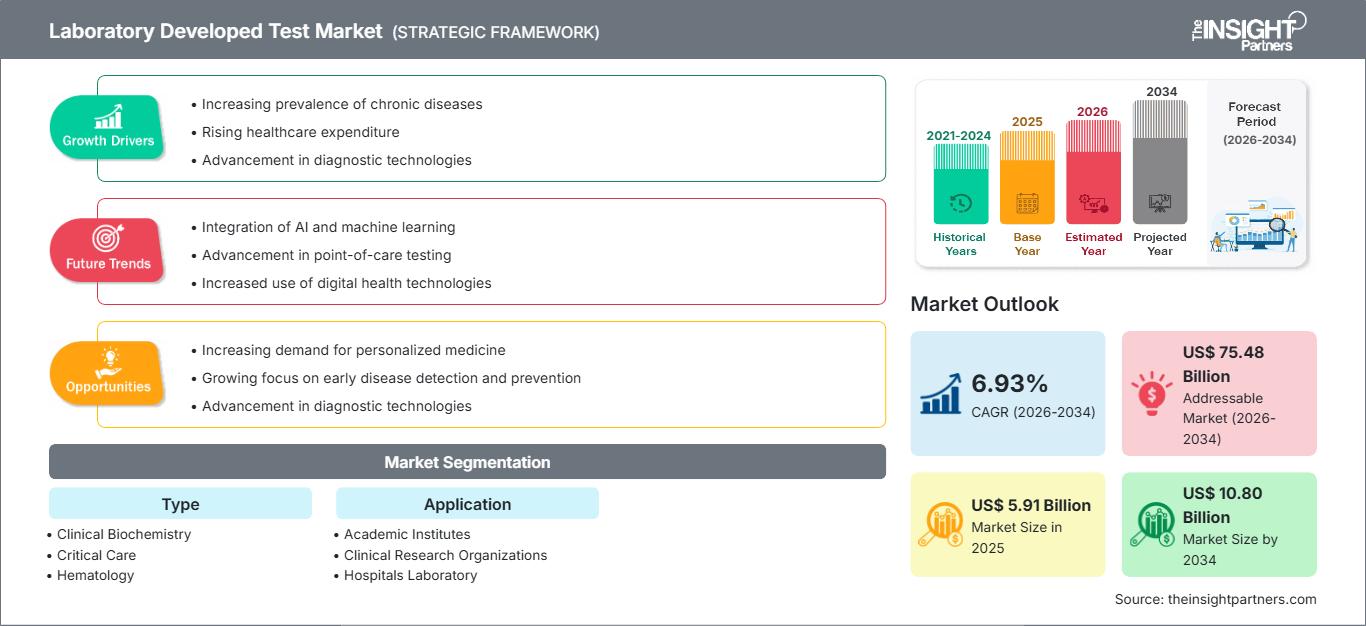
The laboratory developed test (LDT) market size is expected to reach US$ 10.80 Billion by 2034 from US$ 5.91 Billion in 2025. The market is anticipated to register a CAGR of 6.93% during 2026–2034.
Laboratory Developed Test Market Analysis
The laboratory developed test market forecast indicates consistent growth driven by rising demand for personalized medicine, the increasing burden of chronic and rare diseases, rapid advancements in genetic and molecular testing technologies, and the expanding role of diagnostic laboratories in precision healthcare. LDTs offer speed, flexibility, and customizable diagnostic capabilities, enabling laboratories to develop specialized assays where FDA-cleared or commercially available tests may not exist.
Market expansion is also supported by improvements in next-generation sequencing (NGS), in-vitro diagnostics (IVD) automation, and bioinformatics platforms that enable laboratories to design, validate, and deploy sophisticated diagnostic assays. Additionally, evolving regulatory frameworks, especially in the US and Europe, are prompting laboratories to adopt compliant development workflows and invest in quality management systems.
Laboratory Developed Test Market Overview
Laboratory Developed Tests (LDTs) are in-house diagnostic assays designed, manufactured, and used within a single clinical laboratory. These tests are essential for addressing unmet diagnostic needs in areas where no commercially available tests exist or where rapid customization is required.
LDTs support clinical decision-making across multiple specialties, including oncology, infectious diseases, cardiology, genetic disorders, and reproductive health. They allow laboratories to deliver timely, precise, and patient-specific diagnostic results, an increasingly important requirement in personalized medicine. By enabling laboratories to innovate and respond to emerging diseases rapidly, LDTs are becoming integral to modern diagnostic ecosystems.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONLaboratory Developed Test Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Laboratory Developed Test Market Drivers and Opportunities
Market Drivers:
- Rising Demand for Personalized and Precision Medicine: The global shift toward personalized medicine is a major force behind LDT adoption. Clinicians increasingly rely on molecular and genetic diagnostic tools to tailor treatment plans. LDTs provide the flexibility needed to design specialized assays for individualized care.
- Growing Prevalence of Chronic and Rare Diseases: The rising incidence of cancer, inherited disorders, autoimmune conditions, and infectious diseases necessitates advanced diagnostic platforms. LDTs enable early detection, therapy selection, and monitoring, fueling market demand.
- Advancements in Genomics, NGS, and Bioinformatics: Technological advancements, especially in sequencing, multiplex PCR, and proteomics, are enabling laboratories to create high-complexity tests with superior accuracy. These innovations accelerate LDT development cycles.
- Evolving Regulatory Landscape Encouraging Quality Improvement: Regulatory frameworks such as CLIA in the US and IVDR in Europe are prompting laboratories to standardize development processes and enhance test quality. This focus on compliance is increasing investment in LDT development globally.
Market Opportunities:
- Expansion in Emerging Markets With Rapidly Evolving Healthcare Infrastructure: Countries such as China, India, Brazil, and the Gulf region are investing heavily in diagnostic capabilities. This creates opportunities for advanced LDT adoption in oncology, genetics, and infectious diseases.
- Integration of AI/ML for Result Interpretation and Workflow Automation: AI-driven analytics enable faster and more accurate interpretation of genomic and molecular test data. This transforms LDT capabilities by supporting high-throughput, data-intensive analysis.
- Increasing Use of LDTs for Infectious Disease Surveillance and Outbreak Response: During health emergencies, such as viral outbreaks, LDTs can be rapidly developed and deployed, providing diagnostic solutions faster than commercially available tests.
- Opportunities for High-Complexity CLIA-Certified Labs to Expand Test Menus: Large diagnostic laboratory networks can expand their offerings by developing proprietary LDT panels, enhancing competitiveness and revenue opportunities.
Laboratory Developed Test Market Report Segmentation Analysis
The laboratory developed test market share is analyzed across various segments for clarity on structure, growth potential, and emerging trends.
By Type:
- Clinical Biochemistry
- Critical Care
- Hematology
- Microbiology
- Molecular Diagnostics
- Immunology
By Application:
- Academic Institutes
- Clinical Research Organizations
- Hospitals Laboratory
- Specialty Diagnostic Centers
By Geography:
- North America
- Europe
- Asia Pacific
- South & Central America
- Middle East & Africa
Laboratory Developed Test Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 5.91 Billion |
| Market Size by 2034 | US$ 10.80 Billion |
| Global CAGR (2026 - 2034) | 6.93% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Laboratory Developed Test Market Players Density: Understanding Its Impact on Business Dynamics
The Laboratory Developed Test Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Laboratory Developed Test Market Share Analysis by Geography
North America
- Market Share: Largest share globally due to advanced molecular diagnostics infrastructure and high adoption of precision medicine.
- Key Drivers:
- Widespread CLIA-certified laboratory ecosystem
- Early adoption of NGS and high-complexity testing
- Strong focus on oncology and genetic diagnostics
- Trends: Increasing adoption of AI-enabled LDT workflows and expansion of decentralized testing.
Europe
- Market Share: Significant share driven by stringent quality standards and the implementation of IVDR.
- Key Drivers:
- Demand for compliant, high-quality laboratory assays
- National genomics programs
- Growth in rare disease diagnostics
- Trends: Increasing adoption of interoperable diagnostic platforms for seamless patient data exchange.
Asia Pacific
- Market Share: Fastest-growing region.
- Key Drivers:
- Government-supported genomics and precision medicine initiatives
- Growing prevalence of cancer, genetic disorders, and infectious diseases
- Rapid infrastructure expansion
- Trends: AI-driven molecular diagnostics and localized testing solutions for diverse populations.
South & Central America
- Market Share: Growing with the modernization of laboratory systems.
- Key Drivers:
- Public-private partnerships
- Need for cost-effective diagnostic solutions.
- Expansion of private diagnostic laboratories
- Trends: Cloud-enabled interpretation software and cost-efficient LDT workflows.
Middle East & Africa
- Market Share: Developing but rapidly improving.
- Key Drivers:
- National precision medicine strategies
- Growth in advanced oncology and genetic testing capabilities
- Investments in diagnostic infrastructure
- Trends: Integration of LDTs into broader digital health and public health surveillance platforms.
Laboratory Developed Test Market Players Density: Impact on Business Dynamics
Competitive strategies focus on:
- Expansion of proprietary oncology and genetic test portfolios
- Integration of NGS, AI, and bioinformatics into LDT development
- Rapid development of infectious disease assays for public health needs
- Partnerships between laboratory networks and technology vendors
Opportunities & Strategic Moves
- Investment in automated sequencing and molecular testing platforms
- Collaboration with biotech companies to co-develop advanced diagnostic panels
- Expansion into emerging regions with rising diagnostic demand
Major Companies Operating in the Laboratory Developed Test Market
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD.
- QIAGEN
- Illumina, Inc.
- Eurofins Scientific
- Biodesix
- Adaptive
- Biotechnologies Biotheranostics
- Rosetta Genomics Ltd.
Other Companies Analyzed During the Course of Research:
- PerkinElmer Inc.
- Thermo Fisher Scientific Inc.
- Roche Diagnostics
- GeneDx
- 10x Genomics
- Natera Inc.
- Exact Sciences Corporation
- Agilent Technologies
- Helix OpCo LLC
Laboratory Developed Test Market News and Recent Developments
- Quest Diagnostics expanded its oncology portfolio by launching new NGS-based LDT panels for solid tumors and hematologic malignancies to support precision oncology initiatives.
- LabCorp announced the validation of multiple AI-assisted pathology LDTs, improving diagnostic accuracy and turnaround times in cancer detection and classification.
- Mayo Clinic Laboratories introduced new rare disease LDT panels, enabling rapid diagnosis through high-complexity sequencing methods.
- Fulgent Genetics expanded infectious disease LDT offerings, including rapid respiratory and viral panels, strengthening its public health testing capabilities.
Laboratory Developed Test Market Report Coverage and Deliverables
The "Laboratory Developed Test Market Size and Forecast (2021–2034)" report provides a detailed analysis covering:
- LDT market size and forecast (global, regional, country-level)
- Market trends, drivers, restraints, and opportunities
- Detailed PEST and SWOT analysis
- Competitive landscape, concentration analysis, and market positioning
- Regulatory landscape (CLIA, IVDR, global frameworks)
- Company profiles, product portfolios, and strategic developments
Frequently Asked Questions
2. High validation and quality assurance costs
3. Shortage of skilled molecular diagnostic professionals
4. Data management and interpretation challenges due to complex results
2. Europe holds a significant share, driven by IVDR implementation.
3. Asia-Pacific is the fastest-growing region.
2. Diagnostic reference labs
3. Academic and research institutions
4. Specialty clinics
5. Public health labs
2. Increasing prevalence of cancer and genetic diseases
3. Advances in NGS, molecular diagnostics, and bioinformatics
4. Evolving regulatory frameworks driving quality compliance
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For