The North America percutaneous mechanical circulatory support devices market is expected to reach US$ 1,749.25 million by 2027 from US$ 645.23 million in 2019; it is estimated to grow at a CAGR of 13.6% from 2020 to 2027.
The increasing geriatric population, increasing prevalence of cardiovascular diseases and shortage of heart donors are the key factors driving the growth of percutaneous mechanical circulatory support devices. However, high costs of these devices and procedures associated with them as well as product recalls, and impact of COVID-19 pandemic on medical device industry is the major factor hindering the Percutaneous Mechanical Circulatory Support Devices market growth in North America.
Percutaneous mechanical circulatory support devices offers an effective and rapid approach to slow the downward spiral of hemodynamic instability among patients suffering with decompensated heart failures and cardiogenic shocks till a more definitive strategy is perceived among patients to recover from these cardiac ailments. The major roles of the percutaneous mechanical circulatory support devices include improving the native cardiac output, reducing ventricular volume and filling pressures, augmenting coronary perfusion, and maintaining vital organ perfusion.
Heart transplantation has been accepted as the best treatment for patients with medically refractory end-stage heart failure. A central fact about organ transplantation, and heart transplantation, in particular, is the scarcity of organs for transplant. Annual figures published by NHS Blood and Transplant in 2015, showed that there is still a vast shortage of organ donors. There are currently 286 patients on the waiting list for a heart transplant, compared with 126 in 2010. The number of people desperately waiting for a heart transplant has more than doubled in just five years, according to the latest statistics. According to a report published by Ottawa Heart Institute in April 2018, more than 5,000 Canadians could benefit from a heart transplant. However, only 170 transplants were performed annually in Canada due to chronic heart donor shortage.
Patients with advanced heart failure have a poor prognosis, and a heart transplant is still the best treatment option. However, the scarcity of donors, long waiting times, and an increasing number of unstable patients have favored the development of mechanical circulatory support.
Moreover, according to the Heart Failure Society of America, nearly 5.7 million Americans are suffering from heart failure. The data also suggests that almost 1.4 million persons with CHF are under 60 years of age. The Centers for Disease Control and Prevention (CDC) reports that about half of people who develop heart failure die within five years of diagnosis. The cost of heart failure in the US is estimated to be US$ 30.7 billion each year. According to the Mount Sinai Heart Center, every day in America, 33 people die in need of new organs, and about 115,000 people are languishing on waiting lists.
The chronic shortage of heart donors has also led to extend the age limits of donors in certain areas. Thus, the solution to this shortage of heart donors served by the temporarily used percutaneous mechanical circulatory support devices that are also helpful in long-term support for patients suffering from heart failures is likely to be adopted at higher rates in the coming years.
North America is witnessing the growing number of COVID-19 cases. Due to coronavirus spreading, many cities are shutting down, causing treatments and doctors/cardiologists appointment cancellation. Companies operating in the percutaneous mechanical circulatory support devices have limited their production of percutaneous mechanical circulatory support devices. As the treatment procedures are directly contacted with patients’ oral fluid that has a possible risk of infections, for the preventing the spread of COVID-19 it is requested to maintain social distancing as there is a greater risk of getting infected with coronavirus due to direct exposure to oral fluids of the patients. Thus, owing to these factors, the increased production for diagnostics test kits and ventilators are likely to hinder the growth of the percutaneous mechanical circulatory support devices market.
Mexico Percutaneous Mechanical Circulatory Support Devices Market, Revenue and Forecast to 2027 (US$ Mn)
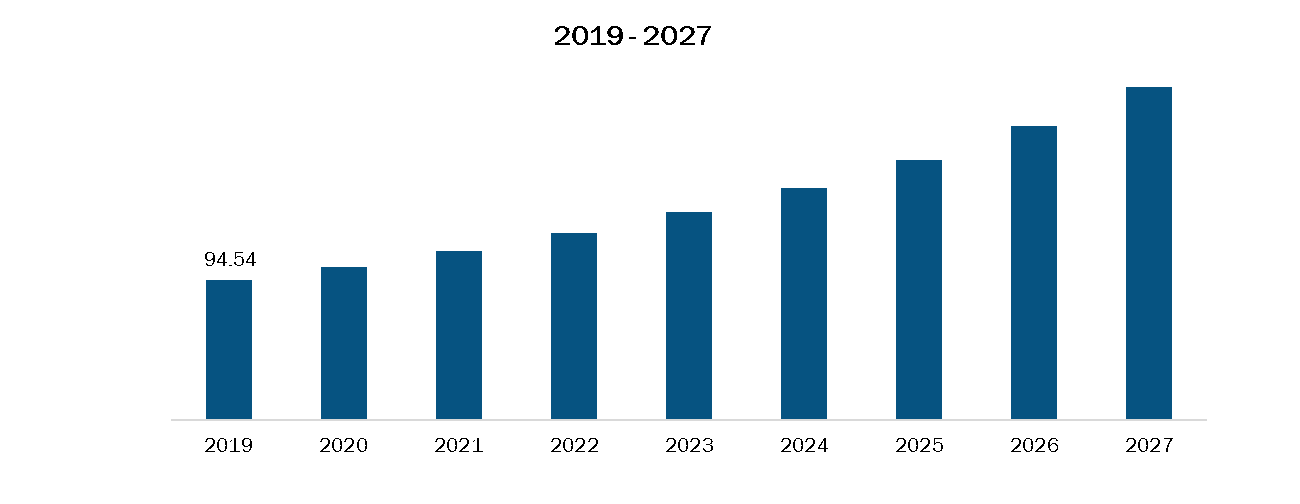
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
NORTH AMERICA PERCUTANEOUS MECHANICAL CIRCULATORY SUPPORT DEVICES MARKET SEGMENTATION
By Product
- Intra-Aortic Balloon Pumps
- VA-Extracorporeal Membrane Oxygenation (ECMO)
- Short-Term Ventricular Assist Devices (VADs)
- Impella
- Tandem Heart
By End User
- Hospitals
- Ambulatory Surgical Centers
- Others
By Country
- US
- Canada
- Mexico
Company Profiles
- Abbott
- Teleflex Incorporated
- Abiomed
- Jarvik Heart, Inc
- Medtronic
North America Percutaneous Mechanical Circulatory Support Devices Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 645.23 Million |
| Market Size by 2027 | US$ 1,749.25 Million |
| CAGR (2020 - 2027) | 13.6% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For