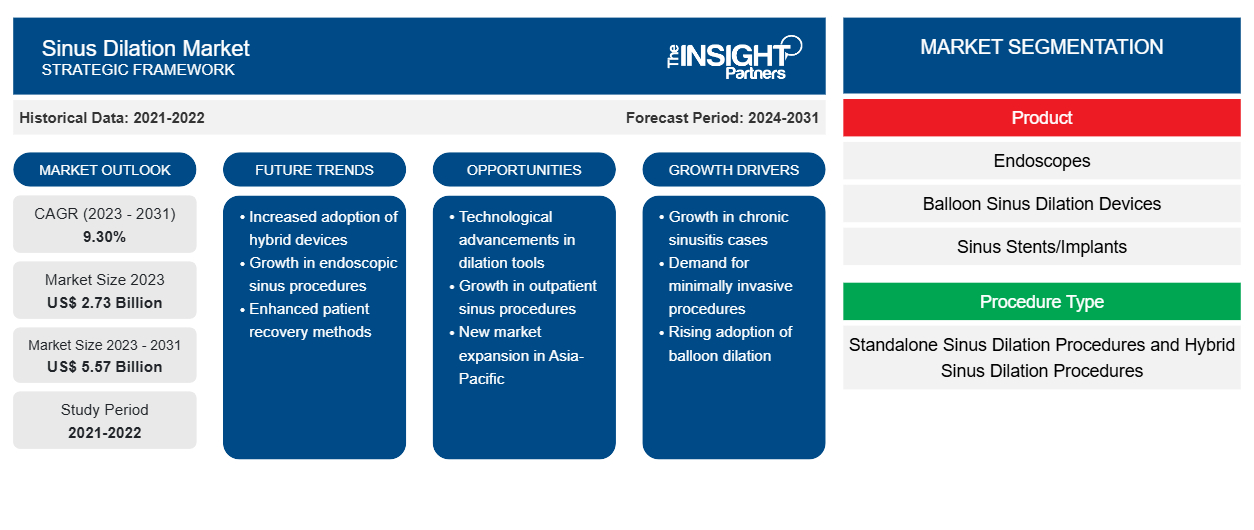
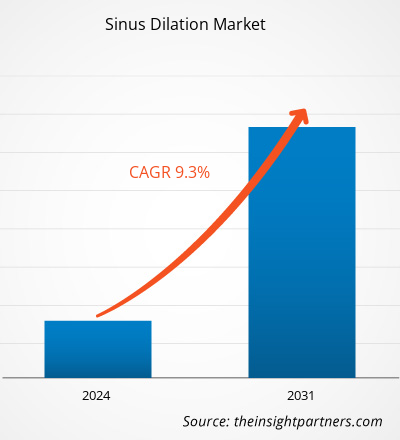
The Sinus Dilation Market size is projected to reach US$ 5.57 billion by 2031 from US$ 2.73 billion in 2023. The market is expected to register a CAGR of 9.30% in 2023–2031. The rising popularity and demand for minimally invasive surgeries owing to their benefits such as less scarring, smaller incisions, high accuracy and decreased risk of complications, shorter hospital stays, and others, coupled with the rising burden of sinusitis globally.
Sinus Dilation Market Analysis
The increasing number of minimally invasive procedures, rising awareness about the advantages of balloon sinuplasty over other conventional techniques, and the prevalence of chronic sinusitis are the major factors fostering market growth. For instance, according to the article published by the University of Cincinnati in February 2024, chronic sinusitis, also known as chronic rhinosinusitis (CRS), affects approximately 14.6% of the United States population. One of the advanced sinus dilation procedures is balloon sinus dilation, a minimally invasive procedure that replaces the traditional sinus surgery procedure. Hence, the increasing number of balloon sinus dilation procedures in the US is another driving factor.
Sinus Dilation Market Overview
North America is the largest market for Sinus Dilation market growth with the US holding the largest market share followed by Canada. Factors such as the presence of major manufacturers of sinus dilation devices, the rising burden of sinusitis and related diseases, the launch of new products by these manufacturers, and the increased burden of risk factors causing sinusitis are contributing to the market growth. One of the most common reasons for patient visits in the US is sinusitis, also known as rhinosinusitis. For instance, according to the data published by CDC in 2022, the total number of balloon sinuplasty procedures performed in the US was 196,316 in 2022. Hence, the increasing number of these procedures is anticipated to propel the market growth.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Sinus Dilation Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Sinus Dilation Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Sinus Dilation Market Drivers and Opportunities
Rising Prevalence of Sinus Disorders Demanding for Sinus Dilation Favors the Market
As per an article published in PubMed in August 2022, chronic sinusitis is known to intensify asthma and, increase morbidity and mortality and severe sinusitis infections lead to complicated asthma cases. Additionally, in the January 2022 data retrieved from the CDC states that around 28.9 million adults were diagnosed with sinusitis, which is about 11.6% of the country's total adult population of the US. Furthermore, approximately, 2.7 million visits are made to physician offices with chronic sinusitis. Thus, the increasing burden of sinusitis is anticipated to be the major stimulating factor for the growth of sinus dilation globally.
Technological Upgradation in the Sinus Dilation Devices– An Opportunity in the Sinus Dilation Market
Technological advancements in sinus dilation devices are a boosting factor for market growth. Moreover, increasing preference for minimally invasive procedures and advances in sinuscope technology are anticipated to boost segmental growth. For instance, in 2022, SPIGGLE & THEIS Medizintechnik updated its dilation device TubaVent shortwide, a single-use insertion instrument with an added catheter. Such advancement in dilation devices is anticipated to drive the overall market growth of the sinus dilation market.
Sinus Dilation Market Report Segmentation Analysis
Key segments that contributed to the derivation of the Sinus Dilation Market analysis are product, procedure, and end users.
- Based on product, the Sinus Dilation market is segmented into Endoscopes, Balloon Sinus Dilation Devices, Sinus Stents/Implants, and Handheld Instruments. The balloon sinus dilation held a larger market share in 2023. The major share can be attributed to advantages such as low risk of bleeding, no intended damage of sinus tissues, etc.
- By procedure, the market is segmented into Standalone Sinus Dilation Procedures and Hybrid Sinus Dilation Procedures. The Standalone Sinus Dilation Procedures segment held the largest share of the market in 2023. The rising adoption of these procedures for the geriatric population due to lesser surgical complications is driving the growth of this segment.
- By end user, the market is segmented into Hospitals, ENT Clinics, and Ambulatory Surgical Centers. The hospitals segment held the major market share in the year 2023.
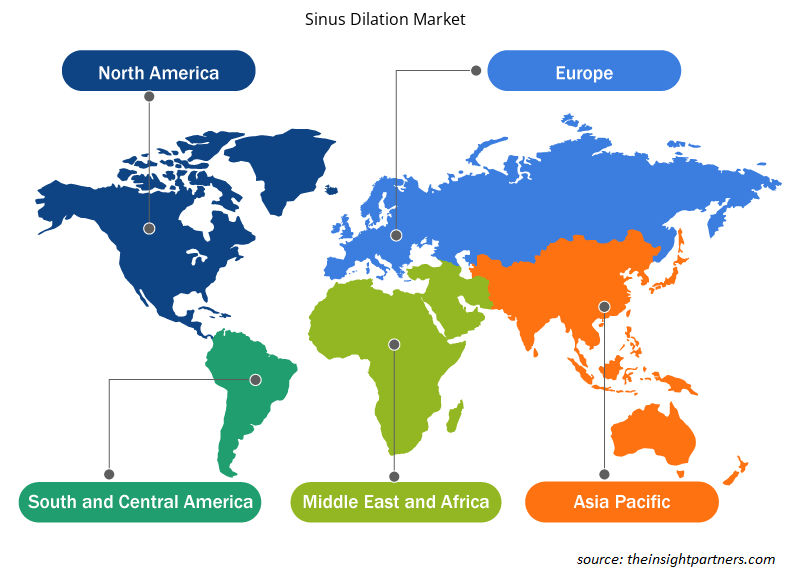
Sinus Dilation Market Share Analysis by Geography
The geographic scope of the Sinus Dilation Market report is mainly divided into five regions: North America, Asia Pacific, Europe, Middle East & Africa, and South America/South & Central America.
North America has dominated the Sinus Dilation Market. The market growth can be attributed to countries. Factors such as the increasing number of balloon dilation procedures, a type of minimally invasive procedure, technological advancement in sinus dilation devices, and the rising prevalence of sinusitis in the North American region. Balloon dilation procedure continues to rise in the US. The procedure used for the treatment of CRS refractory to conservative medical therapy has increased over the last decade. Therefore, the rising number of balloon dilation procedures is propelling the market growth of sinus dilation.
Sinus Dilation Market Regional Insights
The regional trends and factors influencing the Sinus Dilation Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Sinus Dilation Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Sinus Dilation Market
Sinus Dilation Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 2.73 Billion |
| Market Size by 2031 | US$ 5.57 Billion |
| Global CAGR (2023 - 2031) | 9.30% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
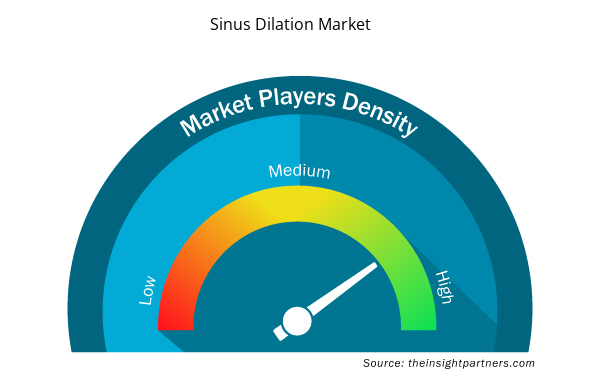
Sinus Dilation Market Players Density: Understanding Its Impact on Business Dynamics
The Sinus Dilation Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Sinus Dilation Market are:
- Acclarent, Inc.
- Aed.MD
- DalentMedical
- Entellus Medical, Inc.
- Innaccel
- Intersect ENT, inc.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Sinus Dilation Market top key players overview
Sinus Dilation Market News and Recent Developments
The Sinus Dilation Market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in the market for Sinus Dilation:
- Acclarent, Inc., part of Johnson & Johnson MedTech leader in developing minimally-invasive Ear, Nose & Throat (ENT) technologies, received clearance from the U.S. Food and Drug Administration (FDA) for the Acclarent AERA Eustachian Tube Balloon Dilation System in the treatment of children, ages 8-17, with persistent obstructive Eustachian tube dysfunction (OETD). Acclarent is the first and only company to receive FDA clearance for Eustachian tube balloon dilation in children. (Source: Acclarent, Inc, Press Release/Company Website/Newsletter, December 2023)
- Medtronic plc, a global leader in healthcare technology, launched the NuVent Eustachian tube dilation balloon, which has been cleared by the U.S. Food and Drug Administration (FDA) for the treatment of chronic, obstructive Eustachian Tube Dysfunction. The NuVent balloon enables surgeons to deliver treatment in an outpatient or office setting. It features a flexible balloon section that allows customized placement based on patient anatomy. (Source: Medtronic plc, Press Release/Company Website/Newsletter, Feb 2022)
- Dalent Medical received additional patent protection for its Sinusleeve balloon device, which further protects the company's proprietary device and method for balloon sinus dilation procedures for treating chronic sinusitis. (Source: Delant Medical, Press Release/Company Website/Newsletter, May 2022)
Sinus Dilation Market Report Coverage and Deliverables
The “Sinus Dilation Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering below areas:
- Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Key future trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Global and regional market analysis covering key market trends, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product, Procedure Type, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
 Get Free Sample For
Get Free Sample For