Traumatic Brain Injury Diagnostics Equipment Market Trends and Analysis by 2030
Traumatic Brain Injury Diagnostics Equipment Market Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technique (Noninvasive, Invasive, and Combination Techniques), Device Type (Imaging Devices and Monitoring Devices), End User (Hospitals & Clinics, Diagnostic Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Historic Data: 2020-2021 | Base Year: 2022 | Forecast Period: 2023-2030- Report Date : Feb 2024
- Report Code : TIPRE00018814
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 305
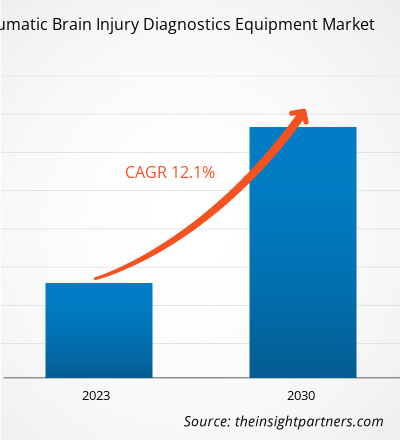
[Research Report] The traumatic brain injury diagnostics equipment market is projected to grow from US$ 3.157 billion in 2022 to US$ 7.872 billion by 2030; the market is estimated to record a CAGR of 12.1% during 2022–2030.
Market Insights and Analyst View:
Factors such as the accelerated demand for advanced diagnostic equipment and quick and effective diagnosis for TBI patients propel the traumatic brain injury diagnostics equipment market growth. However, the adverse effects of contrast medium/agent impede the growth of the market.
Growth Drivers:
Accelerated Demand for Advanced Diagnostic Equipment Drives Traumatic Brain Injury Diagnostics Equipment Market Growth
According to the Medical Research Council 2022 report, 10 million people across the world sustain traumatic brain injury (TBI) annually. Likewise, the Headway 2024 report revealed that acquired brain injury (ABI) is rising in the UK; a total of 356,699 hospital admissions were registered due to ABI in the UK from 2019 to 2020. Among these, the male population was 1.5 times higher than females admitted to hospitals for a head injury. As per The Economist Intelligence Unit report, the global healthcare burden of TBI is estimated to be around US$ 400 billion annually. The most common form of ABI is TBI due to an accident or stroke. The Centers for Disease Control and Prevention (CDC) report revealed that an estimated 1.7 million TBI-related emergency department visits, hospitalizations, and deaths occur annually in the US, especially among adults aged 75 years and older as they are at high risk of falling due to problems with gait and balance. Also, road accidents are the leading cause of TBI-related deaths in the US and are highest among adults aged 20–24 years. Therefore, manufacturers are developing innovative products to diagnose TBI. In October 2023, bioMérieux announced Conformité Européenne (CE) marking for "VIDAS TBI (GFAP, UCH-L1)," a test intended to improve the assessment of patients suffering from mild traumatic brain injury (mTBI). VIDAS TBI (GFAP, UCH-L1) test measures the concentration of glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase L1 (UCH-L1)—the two brain biomarkers released into the bloodstream starting from the first hour following a brain injury. It is an easy-to-interpret test providing a test window of up to 12 hours after injury, which can help in shortening total emergency department workup time. The product's commercial launch was in 2023 for selected European, North African, and South American markets; the global launch is planned for 2024 or 2025.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONTraumatic Brain Injury Diagnostics Equipment Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The “traumatic brain injury diagnostics equipment market analysis” is drawn by considering the following segments: technique, device type, and end user.
Segmental Analysis:
By technique, the traumatic brain injury diagnostics equipment market is segmented into noninvasive, invasive, and combination techniques. The invasive segment held the largest market share in 2022. The non-invasive segment is anticipated to register the highest CAGR of 12.8% during the forecast period.
Noninvasive ultrasound technology is used to efficiently screen the severity of traumatic brain injury (TBI). The EU-funded project "BRAINSAFE" introduced an easy-to-use, handheld, noninvasive absolute intracranial pressure (aICP) meter. This device is an effective alternative to painful, invasive procedures used to assess intracranial injuries. The aICP meter utilizes Doppler ultrasound technology to evaluate blood flow parameters in the ophthalmic artery with the help of a disposable pressure cuff and head frame.
The traumatic brain injury diagnostics equipment market, by device type, is bifurcated into imaging devices and monitoring devices. The imaging devices segment held a larger market share in 2022 and is anticipated to register a considerable CAGR of 11.8% during the forecast period.
The traumatic brain injury diagnostics equipment market, by end user, is categorized into hospitals & clinics, diagnostic centers, and others. The hospitals & clinics segment held the largest market share in 2022 and is anticipated to register a CAGR of 12.0% during the forecast period.
Emergence of Portable Nonivasive Monitoring Devices to Diagnose TBI Patients Acts as a Future Trend in Traumatic Brain Injury Diagnostics Equipment Market
New noninvasive methods for monitoring tissue metabolism can help improve the diagnosis and monitoring of brain conditions such as concussions, stroke, and TBI, owing to which patients can recover more quickly. The consequences of TBI resulted in increasing healthcare burden costs, accounting for US$ 76.5 billion annually, as per the BrainScope company white paper. The installation of "BrainScope One" in less expensive care settings—e.g., emergency department and community settings (including urgent care centers)—may result in a significant reduction of healthcare costs by up to 32.2%, as per the findings revealed in the white paper. BrainScope One aids in eliminating unnecessary CT scans, thereby reducing healthcare costs for TBI.
Further, a team of researchers from the University of Michigan developed a cost-effective, portable, noninvasive tool—Super-Continuum Infrared Spectroscopy of Cytochrome C-Oxidase (SCISCCO) system—to detect neuronal dysfunction. This tool is extremely versatile, having a range of uses from serving as a new device for screening concussion patients to use in the intensive care unit and gauging patients' organ response to treatment.
Likewise, the University of Birmingham 2024 report revealed that researchers from the University of Birmingham have designed and developed the eye-safe device (EyeD)—a novel diagnostic device to detect TBI. It is based on Raman spectroscopy, an optical technique performed simultaneously on fundus imaging and spectroscopic analysis using a smartphone camera. The Raman spectra collected by EyeD from the retina and optic nerves help in analyzing the presence of TBI-specific biochemical changes using the artificial neural network algorithm "SKiNET" as a decision support tool. EyeD is quick, accurate, and noninvasive, causes no additional discomfort, and provides information on the severity of the trauma instantly, owing to which it is highly suitable while using on-site—at the roadside of the unfortunate event or on the sports pitch—to assess TBI.
Regional Analysis:
The scope of the global traumatic brain injury diagnostics equipment market report entails North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2022, North America held the largest traumatic brain injury diagnostics equipment market share. The US is the largest contributor to the market in North America. The Centers for Disease Control and Prevention (CDC) report revealed that an estimated 2.5 million people suffer from TBI annually in the US. According to the KNAPP & ROBERTS report, 1 in every 6 Americans live with TBI-related disability in the US alone, accounting for approx. 5.3 million. With the rising prevalence of TBI, the economic cost accounts for US$ 76.5 billion. Among US$ 76.5 billion, US$ 11.5 billion accounts for direct medical costs and nearly US$ 65 billion for indirect costs. The leading causes of TBI include falls (45%), motor vehicle crashes (14.3%), assaults (10.7%), and unknown (19.0%).
Further, companies are launching innovative products for the diagnosis of TBI. For instance, in August 2022, Abbott announced a new blood test for concussions to predict outcomes from brain injury and treatment interventions. The researchers used Abbott's "i-STAT TBI Plasma Test," the only FDA-cleared rapid test intended for concussion, and Abbott's core laboratory ARCHITECT instrument to measure two biomarkers in blood plasma associated with brain injury. The i-STAT TBI Plasma test measures the level of biomarkers released in the bloodstream in response to the brain injury; the level of biomarkers assists in determining the need for a CT scan.
Additionally, Sense Neuro Diagnostics announced clearance to conduct clinical trials for hemorrhage detection. The new trial approved by the FDA Division of Neurosurgical, Neurointerventional, and Neurodiagnostic Devices began in June 2023, including up to 300 patients at 30 US, Canada, and Indian sites. This noninvasive technology has the potential to collect 360 data points within 2.5 seconds to detect brain hemorrhage or stroke type, thereby helping quick response by physicians, emergency department personnels, neuro ICU teams, and military field hospitals assessing and monitoring TBI. Therefore, the traumatic brain injury diagnostics equipment market size is likely to surge due to innovative product launches by US-based companies to improve outcomes of diagnosis of patients suffering from TBI.
Traumatic Brain Injury Diagnostics Equipment
Traumatic Brain Injury Diagnostics Equipment Market Regional InsightsThe regional trends influencing the Traumatic Brain Injury Diagnostics Equipment Market have been analyzed across key geographies.
Traumatic Brain Injury Diagnostics Equipment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 3.16 Billion |
| Market Size by 2030 | US$ 7.87 Billion |
| Global CAGR (2022 - 2030) | 12.1% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Technique
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Traumatic Brain Injury Diagnostics Equipment Market Players Density: Understanding Its Impact on Business Dynamics
The Traumatic Brain Injury Diagnostics Equipment Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Industry Developments and Future Opportunities:
The traumatic brain injury diagnostics equipment market forecast can help stakeholders in this marketplace plan their growth strategies. A few strategic developments by leading players operating in the market are listed below:
- In October 2023, GE Healthcare Technologies Inc announced a collaboration with Imeka to expand the capabilities of MRI for brain imaging and further develop advanced precision medicine for brain health. Under the collaboration, GE Healthcare Technologies Inc has utilized Imeka’s neuroimaging technology, ANIE biomarker platform, which has broader applicabilities in research and clinical care settings across the spectrum of central nervous system diseases and disorders, such as traumatic brain injury, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. The ANIE biomarker platform is integrated with artificial intelligence that identifies and quantifies axonal loss, neuroinflammation, and demyelination. Thus, with the collaboration, GE Healthcare Technologies Inc integrated its BrainWave advanced diffusion processing package with the ANIE biomarker platform. The newly integrated technologies have allowed researchers and clinicians to analyze diffusion MRI signals in the brain in greater detail.
Competitive Landscape and Key Companies:
Besides highlighting the factors impacting the market, the traumatic brain injury diagnostics equipment market report showcases the developments of prominent players. GE HealthCare Technologies Inc, Elekta AB, Integra LifeSciences Holdings Corp, Natus Medical Inc, Raumedic AG, BrainScope Co Inc, Luciole Medical AG, Soterix Medical Inc, Medtronic Plc, Vivonics Inc, NanoDx Inc, Compumedics Ltd, Sense Diagnostics Inc, NeuraSignal Inc, and Neurovigil Inc are among the prominent companies operating in the market. These companies focus on developing new technologies, upgrading existing products, and expanding their geographic presence to meet the growing consumer demand worldwide.
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For