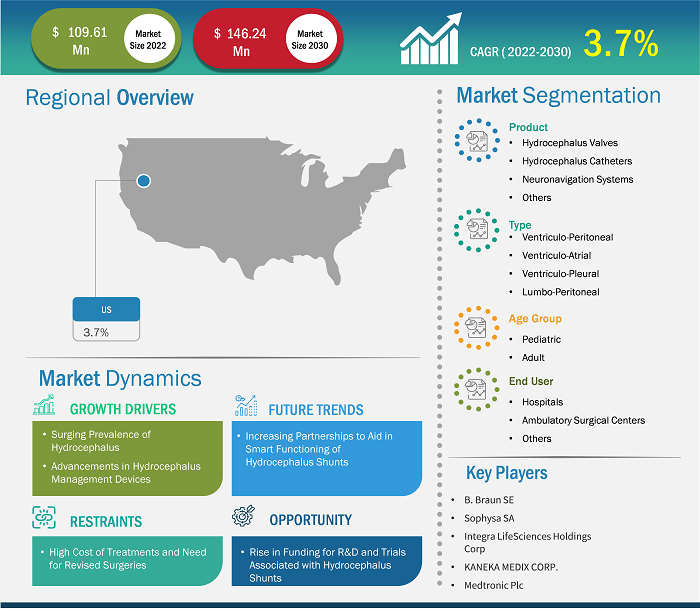
The US Hydrocephalus Shunts market size is expected to reach US$ 146.24 million by 2030 from US$ 109.61 million in 2022. The market is estimated to grow at a CAGR of 3.7% from 2022 to 2030.
Market Insights and Analyst View:
Hydrocephalus is a condition characterized by the buildup of cerebrospinal fluid in the brain, resulting in elevated intracranial pressure. Hydrocephalus shunts, typically implanted via surgical procedures, consist of tube and valve systems that divert excess cerebrospinal fluid from the brain's ventricles to another part of the body, often the abdominal cavity, where it can be reabsorbed. The surging prevalence of hydrocephalus and advancements in hydrocephalus management devices are the key factors driving the market in the US. However, the high cost of treatments and a need for revised surgeries hamper the US hydrocephalus shunts market progress.
In the United States, treatment options for hydrocephalus typically include: Shunt Placement: Shunt systems are commonly used to treat hydrocephalus. A neurosurgeon surgically implants a shunt, which is a device consisting of a tube and a valve, to divert excess cerebrospinal fluid away from the brain to another part of the body where it can be absorbed or reabsorbed.
Endoscopic Third Ventriculostomy (ETV): ETV is another surgical procedure involves creating a small hole in the floor of the third ventricle of the brain to allow cerebrospinal fluid to flow out and be absorbed. ETV is primarily used for certain types of non-communicating hydrocephalus. Ventriculostomy with Catheter: Another surgical option involves inserting a catheter directly into the brain's ventricles to drain excess cerebrospinal fluid.
Revision Surgery: Sometimes, patients with shunts may require revision surgeries to address issues such as blockages, infections, or malfunctions of the shunt system.
Minimally Invasive Techniques: Advancements in minimally invasive procedures, such as endoscopy, have allowed for more precise diagnosis and treatment of hydrocephalus.
Intraventricular Endoscopy: This procedure uses endoscopy to view and treat problems within the ventricular system of the brain.
Monitoring and Adjustments: Regular monitoring of shunt function and intracranial pressure is important. Adjustments to shunt settings may be made to optimize treatment.
Experimental and Research-Based Treatments: In some cases, patients may have the option to participate in clinical trials or experimental treatments being investigated by medical researchers. It's important to note that the choice of treatment depends on various factors, including the type of hydrocephalus, the patient's age, overall health, and individual circumstances. Treatment decisions are typically made in consultation with neurosurgeons and other medical specialists who specialize in the management of hydrocephalus.
Growth Drivers and Challenges:
Hydrocephalus is a chronic neurological disease caused by an abnormal cerebrospinal fluid (CSF) deposition in the cavities (ventricles) of the brain. According to the Hydrocephalus Association, over 1 million people in the US are likely to live with hydrocephalus by 2023, while 1 in 770 babies would develop hydrocephalus yearly. Similarly, as per the article "Navigating the Ventricles: New Insights into the Pathogenesis of Hydrocephalus," published in Elsevier, congenital hydrocephalus occurs in 1 in 500–1,000 babies born in the US. Stroke, intraventricular and subarachnoid hemorrhage, brain tumors, traumatic brain injury, and craniectomy can lead to acquired hydrocephalus. According to a study titled “Management of Hydrocephalus in Children: Anatomic Imaging Appearances of CSF Shunts and Their Complications,” published in the American Journal of Roentgenology in 2020, hydrocephalus affects approximately 1–2% of the US population and results in 70,000 hospitalizations, with the placement of 18,000–33,000 CSF shunts. The annual healthcare expenditures in the country are approximately US$ 2 billion.
The treatment method of hydrocephalus mainly focuses on managing CSF through shunts. There are several forms of treatment for removing excess CSF; the placement of a ventriculoperitoneal (VP) shunt is a common method. The shunts help in removing excess CSF from the brain and then divert it to the other parts of the body, wherein it is absorbed during the circulatory process. According to a study titled “Ventriculoperitoneal Shunts in the Emergency Department,” published in the National Library of Medicine, ~30,000 VP shunt procedures are performed annually in the US. An article published on hydrocephalus and shunts in Ausmed Education states that 33,000 people are implanted with shunts annually in this country. Thus, the high incidence of hydrocephalus drives the demand for hydrocephalus shunts in the US, thereby boosting the US hydrocephalus shunts market growth in this country.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
US Hydrocephalus Shunts Market: Strategic Insights
Market Size Value in US$ 109.61 million in 2022 Market Size Value by US$ 146.24 million by 2030 Growth rate CAGR of 3.7% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Akshay
Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
US Hydrocephalus Shunts Market: Strategic Insights

| Market Size Value in | US$ 109.61 million in 2022 |
| Market Size Value by | US$ 146.24 million by 2030 |
| Growth rate | CAGR of 3.7% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Akshay
Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Report Segmentation and Scope:
The US hydrocephalus shunts market growth is segmented into product, type, age group, and end user. Based on product, the market is segmented into hydrocephalus valves, hydrocephalus catheters, neuronavigation systems, and others. The hydrocephalus valves are further segregated into adjustable pressure valves and fixed pressure valves. In terms of type, the US hydrocephalus shunts market is segmented into is segmented into ventriculoperitoneal, ventriculoatrial, ventriculopleural, and lumboperitoneal. The US hydrocephalus shunts market, by age group, is segmented into pediatric and adults. The US hydrocephalus shunts market, by end user, is segmented into hospitals, ambulatory surgical centers, and others.
Segmental Analysis:
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
The US hydrocephalus shunts market, by product, is segmented into hydrocephalus valves, hydrocephalus catheters, neuronavigation systems, and others. The hydrocephalus valves are further segmented into adjustable and fixed pressure valves. The hydrocephalus valves segment held the largest market share in 2022 and is expected to record a CAGR of 3.9% during 2022-2030.

- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Adjustable or programmable valves regulate intracranial pressure (ICP) based on pressure settings adjusted by a doctor using an external adjustment tool placed outside the body. The settings are changed based on changes needed in CSF draining. Adjustable pressure valves allow professionals to noninvasively change or program valve pressure settings, a major advantage of these valves. A few of these valves are susceptible to adjustment by environmentally solid magnetic fields. These valves ensure greater efficiency in any distribution system via which fluids circulate.
As per a research study published in November 2020 in ScienceDirect, adjustable pressure valves are a valuable option for treating aneurysmal subarachnoid hemorrhage (ASAH) patients undergoing ventriculoperitoneal shunt (VPS) implantation to reduce the need for revision surgery for over or underdrainage. Examples of known adjustable pressure valves include Aesculap, Inc.’s M.blue Adjustable Valve, Codman Certas Plus valve, Integra’s OSV II Flow Regulating Valve, Medtronic plc’s Strata Adjustable Valve, and Sophysa’s Polaris Adjustable Valve.
The US hydrocephalus shunts market, by type, is segmented into ventriculoperitoneal, ventriculoatrial, ventriculopleural, and lumboperitoneal. The ventriculoperitoneal segment held a larger market share in 2022 and the same segment is anticipated to register a higher CAGR during the forecast period. A ventriculoatrial (VA) shunt allows the flow of cerebrospinal fluid (CSF) from the cerebral ventricular system to the atrium of the heart. In the early days, VA shunts were the main treatment for hydrocephalus. Risks associated with VA shunts include thromboembolism, pulmonary hypertension, and shunt nephritis. Another drawback is that its excess catheter tubing cannot be used in the heart as the distal catheter length is fixed. These shunts are also associated with cardiovascular complications. As a result of the abovementioned shortcomings, VA shunts are employed only when all other techniques have failed or are contraindicated.
The US hydrocephalus shunts market, by age group, is segmented into pediatric and adults. The pediatric segment held a largest market share in 2022 and the same segment is anticipated to register a highest CAGR during 2022-2030. The Hydrocephalus Association (HA) estimates that in the US and Canada, 700,000 adults have normal pressure hydrocephalus (NPH), but it is usually misdiagnosed as Alzheimer’s or Parkinson’s disease. Less than 20% of individuals with the disease are properly diagnosed. As per the studies published in Mayfield Brain & Spine, some adult patients treated with a shunt experience a dramatic relief in symptoms, and ~50–80% of patients could expect an improvement in their symptoms in the first 2–3 years post procedure. The Adult Hydrocephalus Clinical Research Network (AHCRN) takes the initiative to create awareness and understanding, accelerate research, and improve treatments for adults with hydrocephalus, thereby contributing to the prevention and cure of hydrocephalus.
The US hydrocephalus shunts market, by end user, is segmented into hospitals, ambulatory surgical centers, others. The hospitals segment held a largest market share in 2022 and the same segment is anticipated to register a highest CAGR during 2022-2030. Ambulatory surgical centers are outpatient or same-day surgery centers where a surgical procedure does not require an overnight stay in the facility, as the procedures performed in these facilities are generally less complicated. The purpose of outpatient surgeries is to save the patient time and keep hospital costs down.
Country Analysis:
The US hydrocephalus shunts market growth is attributed to the surging prevalence of hydrocephalus and advancements in hydrocephalus management devices. Hydrocephalus is a prime cause of morbidity, affecting over 1 million people in the US. The estimated incidence of hydrocephalus is 0.2–0.8 per 1,000 live births. According to the National Institute of Neurological Disorders and Stroke (NINDS), the condition is assumed to affect approximately 1–2 in every 1,000 children born in the US. According to the Los Angeles Health Organization, the University of California, ~125,000 people in the country live with cerebrospinal fluid shunts.
Most hydrocephalus cases are often diagnosed before birth, during delivery or in early childhood. The US hospitals discharge approximately 69,000 patients annually with a primary diagnosis of hydrocephalus, half of which undergo placement of a new shunt. Over half of these admissions were classified as emergent or urgent, and the average cost of treatment was found to be US$ 35,816 per case. The article “Hydrocephalus and Shunts,” published by Ausmed, estimates that 33,000 people undergo shunt placement procedures yearly in the US; CSF diversion surgery and shunt revision surgery account for nearly one-third of all neurosurgical procedures annually in the country.
Competitive Landscape and Key Companies:
Various companies in the US hydrocephalus shunts market focus on organic growth strategies such as launches, expansions, enhancements, and relocations. Inorganic growth strategies in the market were mergers & acquisitions, partnerships, and collaborations. These activities have paved the way for the expansion of businesses and customer base of the US hydrocephalus shunts market players. The companies have maximized their growth with the help of several inorganic strategies to enhance the value and position in the hydrocephalus shunt market.
- In June 2022, CereVasc, Inc. raised US$ 43.9 million in series A financing to support the human trial of the eShunt system.
- In June 2023, Anuncia Medical Inc. announced the successful US commercial launch of the ReFlow Mini Flusher device. This launch has provided neurosurgeons in the US (and soon from other countries) with a new option to reduce the impact of hydrocephalus by providing better access to potentially life-saving therapy for patients suffering from this neurological disorder.
- In May 2020, Aesculap, Inc. launched the M.blue valve, the latest generation of hydrocephalus valve technology. The unique gravitational technology is integrated with a fixed differential pressure unit in one valve, allowing for a simple, position-dependent solution.
- In August 2021, the National Institute of Neurological Disorders and Stroke (NINDS) granted US$ 14 million to researchers at the Johns Hopkins Cerebral Fluid Center, the Department of Neurosurgery, for hydrocephalus treatment studies. The Johns Hopkins Cerebral Fluid Center is conducting the research in partnership with the Adult Hydrocephalus Clinical Research Network (AHCRN). It is coordinated by the Hydrocephalus Association, a nonprofit organization that raises awareness and supports hydrocephalus research. Similarly, in September 2023, Carolyn Harris, associate professor of chemical engineering and materials science at Wayne State University, received a renewal of a research project (RO1) grant totaling $2,666,756 from the National Institutes of Health. The total amount for the entire RO1, from 2016-27, will accumulate to $5,345,009.
- In July 2020, MIT researchers proposed a design to overcome a significant challenge in hydrocephalus catheters — clogging — by leveraging catheter geometry. MIT researchers published their study in the Journal of the Royal Society Interface that proposes and validates a new design for hydrocephalus catheters that pursues to overcome a central challenge of clogging in the design of these devices.
Competitive Landscape
- B. Braun SE
- Sophysa SA
- Integra LifeSciences Holdings Corp
- KANEKA MEDIX CORP.
- Medtronic Plc
- Natus Medical Inc
- Anuncia Inc.
- Desu Medical

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product, Type, Age Group, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Shunt, the surgical insertion of a drainage system is the most common remedy for hydrocephalus. It drains extra cerebrospinal fluid from the brain to another part of the body such as the abdomen, where it can be more easily absorbed. It consists of a long and flexible tube with a valve that keeps fluid from the brain flowing in the right approach and at the proper rate.
The hydrocephalus valves segment held the largest market share of the market in the US hydrocephalus shunts market in 2022.
Key factors that are driving the growth of this market are the surging prevalence of hydrocephalus and growing advancements in hydrocephalus valves.
The CAGR value of the hydrocephalus shunts market during the forecasted period of 2020-2030 is 3.9%.
The hydrocephalus shunts market majorly consists of the players such as B. Braun SE, Sophysa SA, Integra LifeSciences Holdings Corp, KANEKA MEDIX CORP., Medtronic Plc, Natus Medical Inc, Anuncia Inc. and Desu Medical among others.
The List of Companies - US Hydrocephalus Shunts Market
- B. Braun SE
- Sophysa SA
- Integra LifeSciences Holdings Corp
- KANEKA MEDIX CORP.
- Medtronic Plc
- Natus Medical Inc
- Anuncia Inc.
- Desu Medical
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to US Hydrocephalus Shunts Market

Oct 2023
Colonoscopes Market
Forecast to 2028 - COVID-19 Impact and Global Analysis By Product Type (Fiber Optic Colonoscopy Devices, Video Colonoscopy Devices); Application (Colorectal Cancer, Lynch Syndrome, Ulcerative Colitis, Crohn's Disease, Polyp); End User (Hospitals, Ambulatory Surgery Center, Others), and Geography

Oct 2023
Noninvasive Fat Reduction Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Cryolipolysis, Laser Lipolysis, Ultrasound, and Others), End User (Hospitals, Dermatology Clinics & Cosmetic Clinics, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Oct 2023
Medical Ultrasound Flow Meter Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Implementation Type (Clamp-On, Inline, and Others), Technology (Doppler, Transit Time, and Hybrid), Application (Heart and Lung Machines, Extracorporeal Membrane Oxygenation, Perfusion, Organ Transportation Systems, and Others), End User (Hospitals and Clinics, Ambulatory Surgical Centers, Research Laboratories, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Oct 2023
Rapid Test Kits Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Rapid Antigen Testing, Rapid Antibody Testing, and Others), Product (Over-the-Counter Rapid Testing Kit and Professional Rapid Testing Kit), Technology (Lateral Flow Assay, Solid Phase, Agglutination, Immunospot Assay, and Cellular Component-Based), Application (Blood Glucose Testing, Infectious Disease Testing, Pregnancy and Fertility, Cardiometabolic Testing, and Others), End User (Hospital and Clinics, Home Care, Diagnostics Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Oct 2023
Osteoarthritis Therapy Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Therapy Type [Transcutaneous Electrical Nerve Stimulation (TENS), Occupational Therapy, Physical Therapy, Platelet-Rich Plasma Therapy and Stromal Vascular Fraction, Prolotherapy, and Others], Disease Indication (Knee Osteoarthritis, Spine Osteoarthritis, Foot and Ankle Osteoarthritis, Shoulder Osteoarthritis, Hand Osteoarthritis, and Others), End User (Hospitals & Clinics, Specialty Clinics, Ambulatory Surgical Centers, Homecare, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Oct 2023
Bariatric Surgeries Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Type [Adjustable Gastric Bands (AGB), Sleeve Gastrectomy, Gastric Bypass, Biliopancreatic Diversion with Duodenal Switch (BPD-DS), and Others], End User (Hospitals and Ambulatory Surgical Centers), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Oct 2023
Post-Acute Care Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Service Type (Skilled Nursing Facilities, Inpatient Rehabilitation Facilities, Long-Term Care Hospitals, Home Health Agency, and Others), Age (Elderly, Adult, and Others), Disease Conditions (Amputations, Wound Management, Brain Injury and Spinal Cord Injury, Neurological Disorders, and Others), and Geography

Oct 2023
Lung Cancer Therapy Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Therapy Type (Noninvasive and Minimally Invasive), Indication (Non-Small Cell Lung Cancer and Small Cell Lung Cancer), End User (Hospitals, Oncology Clinics, Research Centers, and Others), and Geography (North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa)