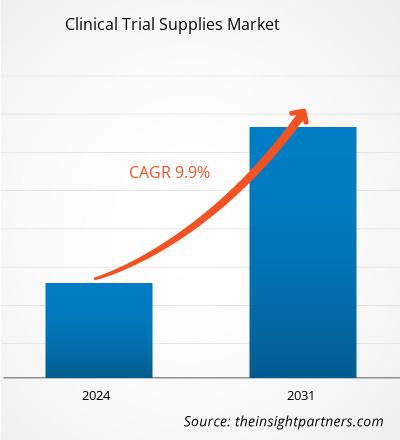
临床试验用品市场规模预计将从 2023 年的 38.1936 亿美元增至 2031 年的 80.8587 亿美元。预计该市场在 2024-2031 年的复合年增长率为 9.9%。随着药物发现成本的增加,临床试验用品变得越来越重要。此外,对开始临床试验的生物制药产品实施更严格的处理要求,临床试验用品战略需要不断改进。全球 临床试验用品市场的增长 归因于关键驱动因素,例如制药和生物制药公司的研发支出增加、临床试验数量的增加以及发达国家药物开发成本的提高。然而,药物开发和临床试验成本的上升以及冠状病毒负面影响给临床试验带来的挑战预计将在预测年份抑制市场增长。全球慢性病的显著增长可能仍然是临床试验用品市场的趋势。
临床试验用品市场分析
亚太地区发展中国家医疗保健行业的蓬勃发展为临床试验用品管理市场参与者拓展业务创造了更好的机会。这些国家庞大的患者群体正在产生更多临床试验的需求。亚太地区进行的临床试验比美国或欧洲多。这种转变归因于低运营成本、巨大的患者招募潜力、合同研究组织的增长、有利的监管环境以及更好的临床试验能力和质量。在北美和欧洲等发达地区,约 35% 的试验因患者招募问题而被推迟,五分之一的试验因受试者不足而无法招募。根据《亚洲临床试验:世界卫生组织数据库研究》报告,2008年至2017年,亚洲临床试验数量年均增长率为:伊朗41.9%、斯里兰卡27.1%、中国23.3%、印度21.3%、日本18.4%、泰国14.7%、马来西亚8.4%、韩国12.9%。
临床试验供应市场参与者数量的增加预计将推动全球临床试验供应市场的发展。
临床试验用品市场概况
临床试验是一项调查研究,旨在确定医疗方法、疗法或设备是否有效、安全且对人类有用。这些研究有助于找出最适合某些疾病的治疗方法。临床试验用品管理对于避免生产过剩、供应过剩和库存过期是必不可少的。
慢性病发病率的上升推动了临床试验用品市场的增长。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
临床试验用品市场:战略洞察
-
获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
临床试验用品市场驱动因素和机遇
慢性病发病率高利好市场
亚洲慢性病发病率高是导致亚洲临床试验数量激增的一个关键因素。根据世界卫生组织 (WHO) 的数据,非传染性疾病导致该地区 55% 的总死亡人数,每年约 800 万人。此外,制药公司可能会分别在中国或韩国进行治疗胃食管癌或 肝癌的药物试验 ,因为这些国家有许多患有这些疾病的患者。此外,亚洲的临床试验费用比美国和欧盟低约 30-40%,因为亚洲国家的看病和医疗费用较低。这一因素预计将增加临床试验,从而促进临床试验用品市场的增长。
外包临床试验服务
将临床试验外包 给合同制造商和服务提供商,为制药商提供了足够的时间来开发其他药物配方,与其他制药公司保持频繁和持续的沟通,并预防风险和其他好处。 Fisher Clinical Services, Inc.、PAREXEL International 和 Piramal Pharma Solutions 等公司为制药和生物制药公司提供物流和配送服务。 因此,上述因素是临床试验用品市场规模增长的原因。
临床试验用品市场报告细分分析
有助于临床试验用品市场分析的关键部分是产品、服务和阶段。
- 根据产品和服务,市场细分为制造、包装和标签以及物流和配送。物流和配送部门在 2023 年占据了最大的市场份额;预计该部门在预测期内将在市场上实现最高的复合年增长率。由于制药行业的合同制造不断增加,物流和配送部门估计是按产品和服务划分的最大部门。临床试验过程所需的成本要高得多。制药和生物制药公司在临床试验期间对药品操作的严格处理投入了大量资金。因此,各种制药公司将其临床试验外包,以避免因生产过剩、供应过剩和库存到期而产生不必要的处理成本。因此,这些因素导致了临床试验供应和物流市场的增长。
- 根据阶段,临床试验用品市场分为 I 期、II 期、III 期和生物等效性研究。III 期部分在 2023 年占据了最大的市场份额,预计在预测期内将在市场上实现最高的复合年增长率。III 期临床试验阶段针对大型患者群体进行。III 期临床试验阶段有助于确定活性药物成分的短期和长期疗效。因此,对配制药物的总体和相关治疗价值进行评估。由于许多患者参与了 III 期临床试验,因此保持药物的有效性、安全性和准确性非常重要,否则可能会导致许多注册 III 期临床试验的患者出现药物不良反应。Shertech Manufacturing 就是这样一家提供 III 期临床试验服务的公司,它为客户提供与疗效和副作用相关的全面、明确的数据。它还协助遵守 FDA 标准,进一步协助将药物引入市场并完成所需的许可申请。因此,疼痛管理对临床试验用品市场做出了贡献,预计在预测期内仍将保持这一趋势。
临床试验用品市场份额(按地区)分析
临床试验用品市场报告的地理范围主要分为五个区域:北美、亚太、欧洲、中东和非洲、南美和中美。
根据地理位置,临床试验用品市场分为五个主要区域:北美、欧洲、亚太地区、南美和中美以及中东和非洲。北美临床试验用品市场基于三个主要国家——美国、加拿大和墨西哥进行分析。预计美国临床试验用品市场在预测期内将占据最大的市场份额。美国临床试验用品市场的增长归因于该地区提供制造、仓储、物流和其他服务的市场参与者的存在,这可能会促进该国的市场增长。例如,Alderley Analytical、Almac 等是知名的制造组织,为 600 多家制药和生物技术公司提供广泛的综合服务。此外,孤儿病和罕见病领域对服务和药品的需求增加预计将鼓励药品制造商开发智能药物并招募患者进行临床试验。该国对新药开发的需求日益增加,预计也将通过跟上制药行业的快速发展步伐来增加行业进入者的数量。
临床试验用品市场区域洞察
Insight Partners 的分析师已详尽解释了预测期内影响临床试验用品市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的临床试验用品市场细分和地理位置。
- 获取临床试验用品市场的区域特定数据
临床试验用品市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2023 年的市场规模 | 38.1936亿美元 |
| 2031 年市场规模 | 80.8587亿美元 |
| 全球复合年增长率(2024 - 2031) | 9.9% |
| 史料 | 2021-2022 |
| 预测期 | 2024-2031 |
| 涵盖的领域 |
按产品和服务
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
临床试验用品市场参与者密度:了解其对业务动态的影响
The Clinical Trial Supplies Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Clinical Trial Supplies Market are:
- Thermo Fisher Scientific
- Catalent
- Eurofins Scientific
- Piramal Pharma Solutions
- PRA Health Sciences
- Marken (a subsidiary of UPS)
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Clinical Trial Supplies Market top key players overview
Clinical Trial Supplies Market News and Recent Developments
The clinical trial supplies market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in the market for innovations, business expansion, and strategies:
- In May 2020, Sharp, part of UDG Healthcare, purchased a pharmaceutical packaging facility from Quality Packaging Specialists International (QPSI) in the US. Situated in Macungie, Pennsylvania, the 160,000ft² facility provides primary and secondary pharmaceutical packaging services to its customers. The Macungie facility provides bottling, blistering, vial labeling, medical device kitting, and sterilization services (Source: Sharp, Press Release)
- In April 2021, Catalent added cryogenic capabilities at its Philadelphia clinical supply services facility. This expansion helped increase Catalent's capabilities in gene therapies, packaging, labeling, and distribution of cryogenic materials for clinical trials (Source: Catalent, Inc., Press Release)
Clinical Trial Supplies Market Report Coverage and Deliverables
The “Clinical Trial Supplies Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering the following areas:
- Clinical Trial SuppliesMarket size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Clinical Trial Supplies Market trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Clinical Trial Supplies market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- 临床试验用品行业格局和竞争分析,涵盖市场集中度、热图分析、知名参与者和最新发展
- 详细的公司简介
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 临床试验用品市场
获取免费样品 - 临床试验用品市场