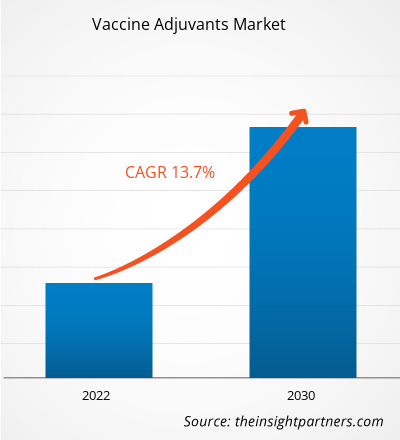
疫苗佐剂市场规模预计将从 2022 年的 24.8239 亿美元增长到 2030 年的 69.3155 亿美元;预计 2022-2030 年期间的复合年增长率为 13.7%。技术进步很可能仍是市场的主要趋势。
疫苗佐剂市场分析
2020 年,NIAID 建立了疫苗佐剂汇编 (VAC),以促进更广泛的科学界与 NIAID 支持的疫苗佐剂研究人员之间的合作。这种基于网络的工具显示佐剂特性,以帮助疫苗制造商确定适合目标疾病的佐剂。此外,通过此类商业策略支持各政府部门促进疫苗佐剂研发、顶级制造商合作和产品发布,可能会为预测期内疫苗佐剂市场的增长提供有利可图的机会。
疫苗佐剂市场概况
疫苗佐剂技术涵盖纳米技术,其生产包括超临界、临界或近临界流体,含或不含乙醇等极性助溶剂[称为超流体 (SFS)]。可生物降解的生物聚合物溶解在 SFS 中,并与亚单位疫苗在水溶液中或作为纳米颗粒浆液混合。因此,正在开发此类新型疫苗佐剂技术,通过新工艺制造可生物降解的聚合物纳米球 (PNS),使疫苗制造商可以排除使用有机溶剂。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
-
获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
疫苗佐剂市场驱动因素和机遇
疫苗佐剂优势凸显 市场青睐
近年来,佐剂疫苗已被开发出来,以增强接种疫苗后的体液和细胞介导免疫反应。通过添加佐剂成分,疫苗效力得到提高。新型佐剂的主要作用机制涉及增强先天免疫系统,并提高人群对感染的体液和细胞介导免疫力。
辅助治疗产品上市及进展
在过去的几十年中,非生物佐剂、生物佐剂及其多类型复合佐剂材料的研究不断增加,旨在为开发可行有效的口服疫苗佐剂提供理论基础。随着与疫苗佐剂相关的研究不断增加,市场也在采取战略举措。例如,2023 年 8 月,HIPRA Human Health 开发的 COVID-19 疫苗 Bimervax 获得了药品和保健产品管理局 (MHRA) 的批准。Bimervax 是 SARS-CoV-2 病毒刺突蛋白的一部分,与佐剂结合,作为旨在引发强烈免疫反应的附加成分。它也可以作为上臂加强针注射,特别是对于 16 岁及以上的人。疫苗佐剂的此类批准可能会有利于 2020 年至 2030 年的市场增长。
疫苗佐剂市场报告细分分析
有助于得出疫苗佐剂市场分析的关键部分是佐剂类别和类型。
- 根据佐剂类别,疫苗佐剂市场分为矿物盐佐剂、乳剂佐剂、脂质体佐剂等。矿物盐佐剂部分在 2022 年占据了较大的市场份额。
- 按类型划分,市场分为人用疫苗佐剂和兽用疫苗佐剂。2022 年,人用疫苗佐剂占据了最大的市场份额。
疫苗佐剂市场份额按地区分析
疫苗佐剂市场报告的地理范围主要分为五个地区:北美、亚太、欧洲、中东和非洲、南美和中美。
北美一直占据市场主导地位,预计未来几年亚太地区将以最高的复合年增长率增长。传染病和大流行病的不断增加促使亚太地区疫苗佐剂的生产加速,从而影响 2022-2030 年的市场增长。在印度,国立卫生研究院 (NIH) 大力支持疫苗的生产。其中一个例子就是由 NIH 资助开发的佐剂,用于增强印度 COVAXIN Alhydroxiquim-II 疫苗的功效。COVAXIN 中使用的佐剂名为“Alhydroxiquim-II”,是由 ViroVax LLC 在实验室中发现和测试的,通过加入一种附着在“Alhydrogel”上的小分子,Alhydrogel 是一种俗称明矾的物质,是人类疫苗中最常用的佐剂。
疫苗佐剂市场区域洞察
Insight Partners 的分析师已详尽解释了预测期内影响疫苗佐剂市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的疫苗佐剂市场细分和地理位置。
- 获取疫苗佐剂市场的区域特定数据
疫苗佐剂市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2022 年市场规模 | 24.8239亿美元 |
| 2030 年市场规模 | 69.3155 亿美元 |
| 全球复合年增长率(2022 - 2030 年) | 13.7% |
| 史料 | 2020-2021 |
| 预测期 | 2022-2030 |
| 涵盖的领域 |
按佐剂类别
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
市场参与者密度:了解其对商业动态的影响
疫苗佐剂市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求又源于消费者偏好的不断变化、技术进步以及对产品优势的认识不断提高等因素。随着需求的增加,企业正在扩大其产品范围,进行创新以满足消费者的需求,并利用新兴趋势,从而进一步推动市场增长。
市场参与者密度是指在特定市场或行业内运营的企业或公司的分布情况。它表明在给定市场空间中,相对于其规模或总市场价值,有多少竞争对手(市场参与者)存在。
在疫苗佐剂市场运营的主要公司有:
- SPI制药公司
- 葛兰素史克公司
- 加拿大移动通信有限公司
- 塞皮克公司
- 夏威夷生物技术公司
- Dynavax技术公司
免责声明:上面列出的公司没有按照任何特定顺序排列。
- 获取疫苗佐剂市场顶级关键参与者概览
疫苗佐剂市场新闻及最新发展
疫苗佐剂市场通过收集一级和二级研究后的定性和定量数据进行评估,其中包括重要的公司出版物、协会数据和数据库。疫苗佐剂市场的一些发展如下所列:
- SPI Pharma Inc 和 Q-Vant Biosciences Inc 合作,将 Q-Vant 在可持续皂苷提取技术方面的领导地位与 SPI 在制药行业的全球影响力和服务专业知识相结合。这包括投资扩大 Q-Vant 专有的 100% 可持续 Q-SAP 技术,以及一项独家商业协议,以加速其皂苷佐剂在全球用于兽医和人类疫苗制剂的采用。(来源:SPI Pharma Inc,新闻稿,2023 年 10 月)
- Seppic 推出了 MONTANIDE GEL P PR,这是一种基于聚合物技术的水性佐剂,专用于禽类注射疫苗,满足了禽类市场对无毒害的需求。此外,MONTANIDE GEL P PR 特别稳定,可以抵抗禽类疫苗中经常使用的不稳定抗原介质。(来源:Seppic,新闻稿,2024 年 5 月)
疫苗佐剂市场报告覆盖范围和交付成果
“疫苗佐剂市场规模和预测(2020-2030 年)”报告对以下领域进行了详细的市场分析:
- 疫苗佐剂市场规模及全球、区域和国家层面所有主要细分市场的预测
- 疫苗佐剂市场趋势,以及驱动因素、限制因素和关键机遇等市场动态
- 详细的 PEST/波特五力分析和 SWOT 分析
- 疫苗佐剂市场分析涵盖主要市场趋势、全球和区域框架、主要参与者、法规和最新市场发展3
- 行业格局和竞争分析,涵盖市场集中度、热图分析、知名参与者以及疫苗佐剂市场的最新发展
- 详细的公司简介
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势























 获取免费样品 - 疫苗佐剂市场
获取免费样品 - 疫苗佐剂市场