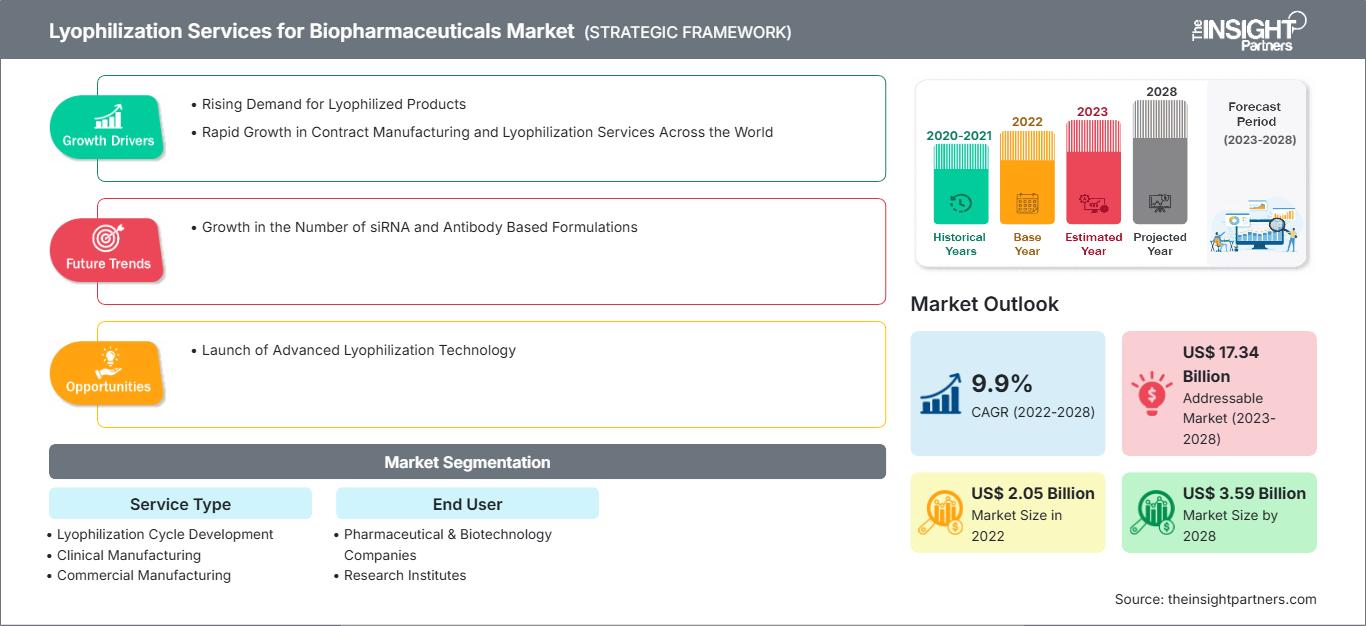
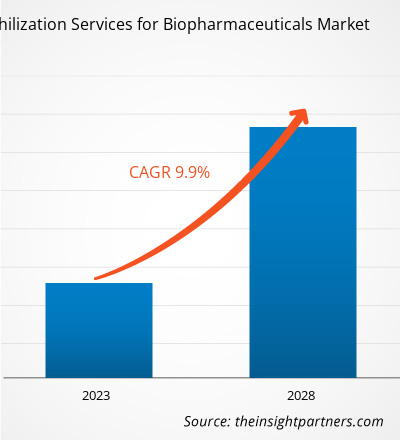
[Informe de investigación] Se espera que el mercado de servicios de liofilización para productos biofarmacéuticos crezca de US$ 2.051,41 millones en 2022 a US$ 3.586,55 millones en 2028; se estima que registrará una CAGR del 9,9% de 2023 a 2028.
El crecimiento del mercado de servicios de liofilización para productos biofarmacéuticos se atribuye a la creciente demanda de productos liofilizados y al rápido crecimiento de los servicios de liofilización y fabricación por contrato en todo el mundo.
El mercado de servicios de liofilización para productos biofarmacéuticos se segmenta según el tipo de servicio, el usuario final y la región. El informe ofrece información y un análisis exhaustivo del mercado, destacando parámetros como las tendencias, los avances tecnológicos, la dinámica del mercado y el análisis del panorama competitivo de los principales actores.
Servicios de liofilización para el mercado biofarmacéutico: análisis del mercado
El rápido aumento de los servicios de fabricación por contrato y liofilización impulsa el crecimiento del mercado de servicios de liofilización para productos biofarmacéuticos.
El mercado biofarmacéutico continúa expandiéndose a nivel mundial, lo que puede ser el principal motor de crecimiento para la industria farmacéutica. La fabricación de productos parenterales biofarmacéuticos se ha expandido recientemente gracias a la aprobación de más productos biológicos terapéuticos. Las empresas biotecnológicas subcontratan sus servicios a organizaciones de fabricación por contrato (OFC) para satisfacer sus necesidades de llenado y acabado y reducir el riesgo de contaminación microbiana. Las empresas biofarmacéuticas dependen de las OFC para obtener la capacidad y las capacidades necesarias; en algunos casos, las OFC representan una parte importante de la producción de una empresa.
Personalice este informe según sus necesidades
Obtendrá personalización en cualquier informe, sin cargo, incluidas partes de este informe o análisis a nivel de país, paquete de datos de Excel, así como también grandes ofertas y descuentos para empresas emergentes y universidades.
Servicios de liofilización para el mercado biofarmacéutico: Perspectivas estratégicas
-
Obtenga las principales tendencias clave del mercado de este informe.Esta muestra GRATUITA incluirá análisis de datos, desde tendencias del mercado hasta estimaciones y pronósticos.
Establecer capacidades y operaciones de liofilización internas requiere equipos y experiencia especializados, lo cual resulta costoso y requiere mucho tiempo. Sin embargo, la externalización resulta más económica y aumenta la eficiencia de los procesos de fabricación. Además, permite a las empresas biotecnológicas redirigir recursos a otras áreas. Así, los desarrolladores de fármacos y las empresas biofarmacéuticas externalizan estas operaciones a CMO para reducir los costes generales de producción y rendimiento. Hace unos años, la industria de CMO era un nicho de mercado de servicios que ofrecía capacidad de fabricación adicional o servicios específicos a las empresas biotecnológicas. Actualmente, muchas empresas biotecnológicas externalizan diversos servicios, desde el desarrollo inicial de fármacos hasta la fabricación a escala comercial. A medida que la industria biotecnológica evoluciona de la producción a gran escala a terapias específicas y de nicho (medicina personalizada), aumenta la demanda de capacidades operativas flexibles, escalas de producción y operaciones multiproducto. Debido a todos estos factores, la preferencia por los CMO está en aumento. Una de estas empresas, que ofrece instalaciones especializadas y líneas dedicadas a las operaciones de liofilización, es Jubilant HollisterStier Contract Manufacturing & Services. El CMO ofrece llenado/acabado estéril de la Fase I mediante un inyectable estéril comercial y una gama completa de servicios de liofilización. Para satisfacer la creciente demanda de sus servicios, Jubilant está instalando un nuevo liofilizador de 385 pies cuadrados. Por lo tanto, la creciente capacidad y disponibilidad de los CMO biofarmacéuticos impulsa el crecimiento del mercado de servicios de liofilización para productos biofarmacéuticos.
Servicios de liofilización para el mercado biofarmacéutico: análisis por tipo de servicio
Según el tipo de servicio, el mercado global de servicios de liofilización para productos biofarmacéuticos se segmenta en fabricación comercial, desarrollo de ciclos de liofilización, fabricación clínica y servicios analíticos de liofilización. El segmento de fabricación comercial registró la mayor cuota de mercado en 2022. Se espera que el segmento de desarrollo de ciclos de liofilización registre la mayor tasa de crecimiento anual compuesta (TCAC) durante el período de pronóstico.
Servicios de liofilización para el mercado biofarmacéutico: análisis basados en el usuario final
Según el usuario final, el mercado global de servicios de liofilización para productos biofarmacéuticos se segmenta en empresas farmacéuticas y biotecnológicas, institutos de investigación y otros. El segmento de empresas farmacéuticas y biotecnológicas tuvo la mayor participación de mercado en 2022. Se espera que el segmento de institutos de investigación registre la mayor tasa de crecimiento anual compuesta (TCAC) durante el período de pronóstico.
Las empresas del mercado de servicios de liofilización para productos biofarmacéuticos adoptan estrategias inorgánicas y orgánicas, como fusiones y adquisiciones. A continuación, se enumeran algunos desarrollos clave recientes del mercado:
- En noviembre de 2022, LTI anunció su participación en el desarrollo, la ingeniería de procesos y la preparación de material clínico para la vacuna candidata contra la tuberculosis ID93/GLA-SE. La vacuna contra la tuberculosis ha superado con éxito la fase 2 de ensayos clínicos. Este estudio representa el primer informe sobre la liofilización exitosa de una vacuna candidata de subunidad termoestable que contiene un adyuvante en emulsión.
- En mayo de 2022, Jubilant HollisterStier LLC celebró un acuerdo de cooperación por US$149,6 millones con el Comando de Contrataciones del Ejército, en coordinación con la Oficina Ejecutiva del Programa Conjunto para la Defensa Química, Biológica, Radiológica y Nuclear (JPEOCBRND) en nombre de la Autoridad de Investigación y Desarrollo Biomédico Avanzado (BARDA), dentro del Departamento de Salud y Servicios Humanos de los EE. UU.
- En octubre de 2021, PCI Pharma Services (PCI) anunció la firma de un acuerdo definitivo para adquirir Lyophilization Services of New England, Inc. (LSNE), una organización líder de desarrollo y fabricación por contrato (CDMO) con sede en Bedford, Nuevo Hampshire, de la firma global de capital privado Permira. La adquisición añade cinco instalaciones aprobadas por la FDA en EE. UU. (Nuevo Hampshire, Wisconsin) y Europa (España), y se espera la aprobación de una sexta en los próximos meses. Además, se están desarrollando tres instalaciones adicionales. Estas instalaciones fortalecerán su red global de 30 centros.
- En julio de 2021, Albany Molecular Research, Inc. (AMRI) anunció que cambiará su nombre a Curia, a partir del 12 de julio de 2021. El nuevo nombre refuerza el posicionamiento estratégico de la empresa como CDMO global de extremo a extremo, aplicando su experiencia científica y amplias capacidades desde investigación y desarrollo (I+D) hasta fabricación comercial para permitir que sus clientes farmacéuticos y biotecnológicos promuevan nuevos productos importantes que mejoren vidas.
Servicios de liofilización para biofarmacéuticos: perspectivas regionales del mercado
Los analistas de The Insight Partners han explicado detalladamente las tendencias regionales y los factores que influyen en el mercado de servicios de liofilización para productos biofarmacéuticos durante el período de pronóstico. Esta sección también analiza los segmentos y la geografía del mercado de servicios de liofilización para productos biofarmacéuticos en América del Norte, Europa, Asia Pacífico, Oriente Medio y África, y América del Sur y Central.
Alcance del informe de mercado de servicios de liofilización para productos biofarmacéuticos
| Atributo del informe | Detalles |
|---|---|
| Tamaño del mercado en 2022 | US$ 2.05 mil millones |
| Tamaño del mercado en 2028 | US$ 3.59 mil millones |
| CAGR global (2022-2028) | 9,9% |
| Datos históricos | 2020-2021 |
| Período de pronóstico | 2023-2028 |
| Segmentos cubiertos |
Por tipo de servicio
|
| Regiones y países cubiertos |
América del norte
|
| Líderes del mercado y perfiles de empresas clave |
|
Servicios de liofilización para productos biofarmacéuticos: Densidad de actores del mercado: comprensión de su impacto en la dinámica empresarial
El mercado de servicios de liofilización para productos biofarmacéuticos está creciendo rápidamente, impulsado por la creciente demanda del usuario final debido a factores como la evolución de las preferencias de los consumidores, los avances tecnológicos y un mayor conocimiento de los beneficios del producto. A medida que aumenta la demanda, las empresas amplían su oferta, innovan para satisfacer las necesidades de los consumidores y aprovechan las tendencias emergentes, lo que impulsa aún más el crecimiento del mercado.
- Obtenga una descripción general de los principales actores del mercado de servicios de liofilización para productos biofarmacéuticos.
Perfiles de empresas - Servicios de liofilización para el mercado biofarmacéutico
- Soluciones médicas ATTWILL
- Axcellerate Pharma LLC
- Laberinto Biofarmacéutico LLC
- Fabricación estéril de Berkshire
- Servicios farmacéuticos PCI
- Curia Global Inc
- Emergent BioSolutions Inc
- Jubilant HollisterStier LLC
- Biofortuna
- Tecnología de liofilización Inc.
- GRUPO SYNERLAB
- Análisis histórico (2 años), año base, pronóstico (7 años) con CAGR
- Análisis PEST y FODA
- Tamaño del mercado, valor/volumen: global, regional y nacional
- Industria y panorama competitivo
- Conjunto de datos de Excel
Informes recientes
Informes relacionados
Testimonios
Razón para comprar
- Toma de decisiones informada
- Comprensión de la dinámica del mercado
- Análisis competitivo
- Información sobre clientes
- Pronósticos del mercado
- Mitigación de riesgos
- Planificación estratégica
- Justificación de la inversión
- Identificación de mercados emergentes
- Mejora de las estrategias de marketing
- Impulso de la eficiencia operativa
- Alineación con las tendencias regulatorias






















 Obtenga una muestra gratuita para - Servicios de liofilización para el mercado biofarmacéutico
Obtenga una muestra gratuita para - Servicios de liofilización para el mercado biofarmacéutico