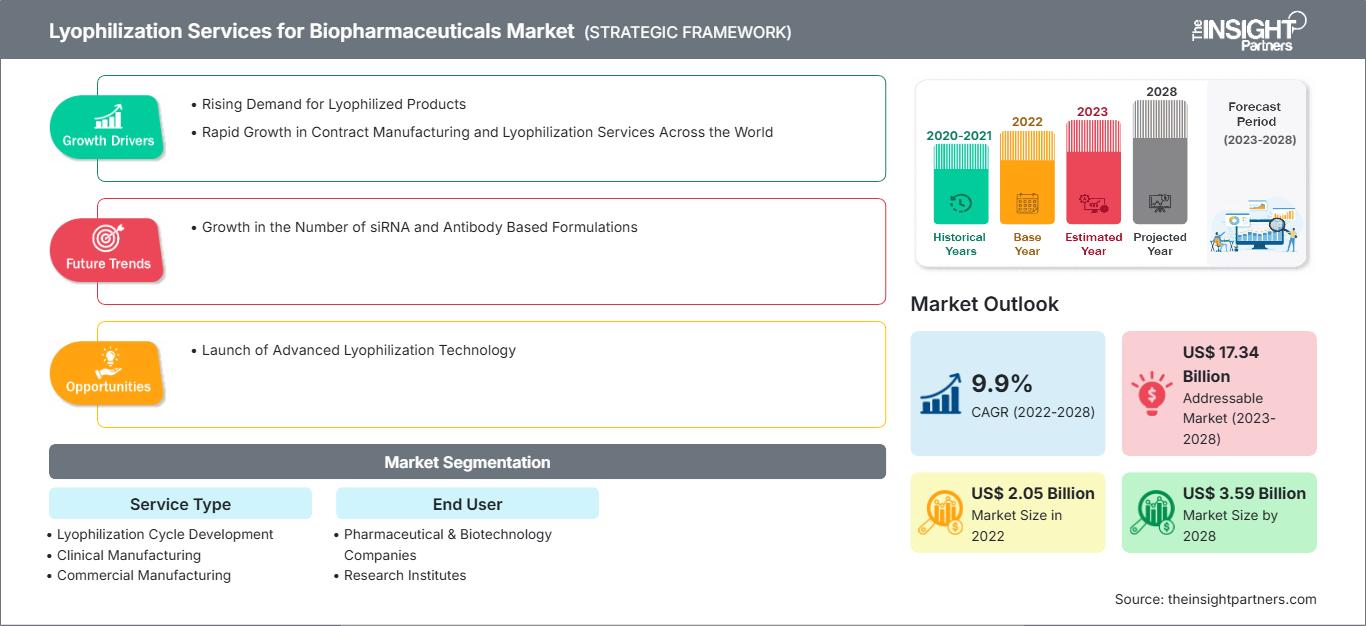
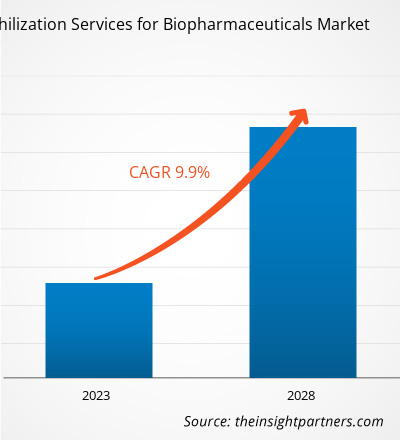
[연구 보고서] 바이오 의약품용 동결건조 서비스 시장은 2022년 20억 5,141만 달러에서 2028년 35억 8,655만 달러로 성장할 것으로 예상되며, 2023년부터 2028년까지 연평균 9.9% 성장할 것으로 예상됩니다.
바이오 의약품용 동결건조 서비스 시장 성장은 동결건조 제품에 대한 수요 증가와 전 세계적으로 급속도로 성장하는 계약 제조 및 동결건조 서비스 덕분입니다.
바이오 의약품용 동결건조 서비스 시장은 서비스 유형, 최종 사용자 및 지역을 기준으로 세분화됩니다. 본 보고서는 바이오 의약품용 동결건조 서비스 시장 동향, 기술 발전, 시장 역학, 주요 시장 참여자들의 경쟁 환경 분석 등의 요소를 중심으로 시장에 대한 통찰력과 심층 분석을 제공합니다.
바이오 의약품 시장용 동결건조 서비스 - 시장 분석
계약 제조 및 동결건조 서비스의 급속한 증가로 바이오 의약품 시장 성장 견인
바이오 의약품 시장은 전 세계적으로 지속적으로 확대되고 있으며, 이는 제약 산업의 주요 성장 동력이 될 수 있습니다. 최근 더 많은 치료용 생물학적 제제가 승인됨에 따라 바이오 의약품 비경구 제조가 확대되었습니다. 생명공학 기업들은 완제품(fill-and-finish) 생산 요건을 충족하고 미생물 오염 위험을 줄이기 위해 계약 제조 기관(CMO)에 아웃소싱을 제공합니다. 바이오 의약품 기업들은 필요에 따라 생산 능력과 역량을 제공하기 위해 계약 제조 기관(CMO)에 의존하고 있으며, 경우에 따라 CMO는 기업 생산량의 상당 부분을 담당하기도 합니다.
요구 사항에 맞게 이 보고서를 사용자 정의하십시오.
이 보고서의 일부, 국가 수준 분석, Excel 데이터 팩을 포함하여 모든 보고서에 대한 사용자 정의를 무료로 받을 수 있을 뿐만 아니라 스타트업 및 대학을 위한 훌륭한 제안 및 할인을 이용할 수 있습니다
생물의약품 시장을 위한 동결건조 서비스: 전략적 통찰력
-
이 보고서의 주요 주요 시장 동향을 확인하세요.이 무료 샘플에는 시장 동향부터 추정 및 예측에 이르기까지 데이터 분석이 포함됩니다.
자체 동결건조 시설 및 운영을 구축하려면 특수 장비와 전문 지식이 필요하며, 이는 비용과 시간이 많이 소요됩니다. 그러나 아웃소싱은 비용이 저렴하고 제조 공정의 효율성을 높입니다. 또한, 생명공학 기업이 자원을 다른 분야로 재분배할 수 있도록 합니다. 따라서 약물 개발업체와 바이오 제약 회사는 전체 생산 및 수율 비용을 절감하기 위해 이러한 운영을 CMO에 아웃소싱합니다. 몇 년 전만 해도 CMO 산업은 생명공학 기업에 추가 생산 능력이나 특정 서비스를 제공하는 틈새 서비스 시장이었습니다. 이제 많은 생명공학 기업들이 초기 약물 개발부터 상업적 규모 생산에 이르기까지 다양한 서비스를 아웃소싱하고 있습니다. 생명공학 산업이 대규모 생산에서 틈새 시장 및 표적 치료(맞춤 의학)로 전환함에 따라 유연한 운영 역량, 생산 규모 및 다제품 운영에 대한 수요가 증가하고 있습니다. 이러한 모든 요인으로 인해 CMO에 대한 관심이 높아지고 있습니다. 동결건조 운영을 위한 전문 시설과 전용 라인을 제공하는 CMO 중 하나가 Jubilant HollisterStier Contract Manufacturing & Services입니다. CMO는 상용 무균 주사제와 다양한 동결건조 서비스를 통해 1상 임상시험의 무균 충전/마무리를 제공합니다. Jubilant는 증가하는 서비스 수요에 대응하기 위해 385제곱피트(약 385제곱피트) 규모의 새로운 동결건조기를 설치하고 있습니다. 따라서 바이오제약 CMO의 역량과 가용성 증가는 바이오제약 시장 성장을 위한 동결건조 서비스의 성장을 촉진하고 있습니다.
바이오제약 시장용 동결건조 서비스 - 서비스 유형 기반 분석
서비스 유형을 기준으로 전 세계 바이오제약용 동결건조 서비스 시장은 상용 제조, 동결건조 사이클 개발, 임상 제조, 동결건조 분석 서비스로 구분됩니다. 상업용 제조 부문은 2022년에 가장 큰 시장 점유율을 기록했습니다. 동결건조 사이클 개발 부문은 예측 기간 동안 가장 높은 CAGR을 기록할 것으로 예상됩니다.
바이오 의약품용 동결건조 서비스 시장 - 최종 사용자 기반 인사이트
최종 사용자를 기준으로, 전 세계 바이오 의약품용 동결건조 서비스 시장은 제약 및 생명공학 회사, 연구소 등으로 세분화됩니다. 제약 및 생명공학 회사 부문은 2022년에 가장 큰 시장 점유율을 기록했습니다. 연구소 부문은 예측 기간 동안 가장 높은 CAGR을 기록할 것으로 예상됩니다.
바이오 의약품용 동결건조 서비스 시장 기업들은 인수합병과 같은 무기적 및 유기적 전략을 채택합니다. 최근 주요 시장 동향은 다음과 같습니다.
- 2022년 11월, LTI는 결핵 백신 후보물질 ID93/GLA-SE의 개발, 공정 엔지니어링 및 임상 물질 준비에 참여한다고 발표했습니다. 이 결핵 백신은 임상 2상 시험에 성공적으로 진입했습니다. 이 연구는 에멀젼 기반 보조제를 함유한 열안정성 서브유닛 백신 후보의 성공적인 동결건조에 대한 최초의 보고서를 나타냅니다.
- 2022년 5월, Jubilant HollisterStier LLC는 미국 보건복지부 산하 생물의학 첨단 연구개발국(BARDA)을 대신하여 화학, 생물, 방사선 및 핵 방어를 위한 합동 프로그램 집행 사무소(JPEOCBRND)와 협력하여 육군 계약 사령부와 1억 4,960만 달러 규모의 협력 계약을 체결했습니다.
- 2021년 10월, PCI Pharma Services(PCI)는 글로벌 사모펀드 회사인 Permira로부터 뉴햄프셔주 베드포드에 본사를 둔 최고의 계약 개발 및 제조 기관(CDMO)인 Lyophilization Servicesof New England, Inc.(LSNE)를 인수하기 위한 최종 계약을 체결했다고 발표했습니다. 이번 인수로 미국(뉴햄프셔, 위스콘신)과 유럽(스페인)에 FDA 승인을 받은 5개의 시설이 추가되었으며, 향후 몇 달 안에 6번째 시설도 승인을 받을 예정입니다. 또한, 3개의 추가 시설이 개발 중입니다. 이 시설들은 30개 시설에 달하는 글로벌 네트워크를 강화할 것입니다.
- 2021년 7월, Albany Molecular Research, Inc.(AMRI)는 2021년 7월 12일부터 사명을 Curia로 변경한다고 발표했습니다. 새로운 사명은 연구 개발(R&D)부터 상업 생산까지, 광범위한 과학적 전문성과 역량을 활용하여 제약 및 생명공학 고객이 삶의 질을 향상시키는 중요한 신제품을 개발할 수 있도록 지원하는 엔드 투 엔드 글로벌 CDMO로서의 전략적 입지를 강화합니다.
생물약품 시장을 위한 동결건조 서비스
The Insight Partners의 분석가들은 예측 기간 동안 바이오의약품용 동결건조 서비스 시장에 영향을 미치는 지역별 동향과 요인을 면밀히 분석했습니다. 이 섹션에서는 북미, 유럽, 아시아 태평양, 중동 및 아프리카, 그리고 중남미 지역의 바이오의약품용 동결건조 서비스 시장 부문 및 지역별 현황도 살펴봅니다.
생물의약품 시장 보고서 범위를 위한 동결건조 서비스
| 보고서 속성 | 세부 |
|---|---|
| 시장 규모 2022 | US$ 2.05 Billion |
| 시장규모별 2028 | US$ 3.59 Billion |
| 글로벌 CAGR (2022 - 2028) | 9.9% |
| 이전 데이터 | 2020-2021 |
| 예측 기간 | 2023-2028 |
| 다루는 세그먼트 |
By 서비스 유형
|
| 포함된 지역 및 국가 |
북미
|
| 시장 선도 기업 및 주요 회사 프로필 |
|
생물의약품 시장 참여자를 위한 동결건조 서비스 밀도: 비즈니스 역학에 미치는 영향 이해
바이오의약품 동결건조 서비스 시장은 소비자 선호도 변화, 기술 발전, 그리고 제품 효능에 대한 인식 제고 등의 요인으로 인한 최종 사용자 수요 증가에 힘입어 빠르게 성장하고 있습니다. 수요 증가에 따라 기업들은 제품 및 서비스 확장, 소비자 니즈 충족을 위한 혁신, 그리고 새로운 트렌드를 적극 활용하며 시장 성장을 더욱 가속화하고 있습니다.
- 을 얻으세요 생물의약품 시장을 위한 동결건조 서비스 주요 주요 플레이어 개요
회사 프로필 - 생물약학 시장을 위한 동결건조 서비스
- ATTWILL Medical Solutions
- Axcellerate Pharma LLC
- Labyrinth Biopharma LLC
- Berkshire Sterile Manufacturing
- PCI Pharma Services
- Curia Global Inc
- Emergent BioSolutions Inc
- Jubilant HollisterStier LLC
- Biofortuna
- Lyophilization Technology Inc.
- SYNERLAB GROUP
- 과거 분석(2년), 기준 연도, CAGR을 포함한 예측(7년)
- PEST 및 SWOT 분석
- 시장 규모 가치/거래량 - 글로벌, 지역, 국가
- 산업 및 경쟁 환경
- Excel 데이터세트
최근 보고서
관련 보고서
사용 후기
구매 이유
- 정보에 기반한 의사 결정
- 시장 역학 이해
- 경쟁 분석
- 고객 인사이트
- 시장 예측
- 위험 완화
- 전략 기획
- 투자 타당성 분석
- 신흥 시장 파악
- 마케팅 전략 강화
- 운영 효율성 향상
- 규제 동향에 발맞춰 대응






















 무료 샘플 받기 - 생물의약품 시장을 위한 동결건조 서비스
무료 샘플 받기 - 생물의약품 시장을 위한 동결건조 서비스