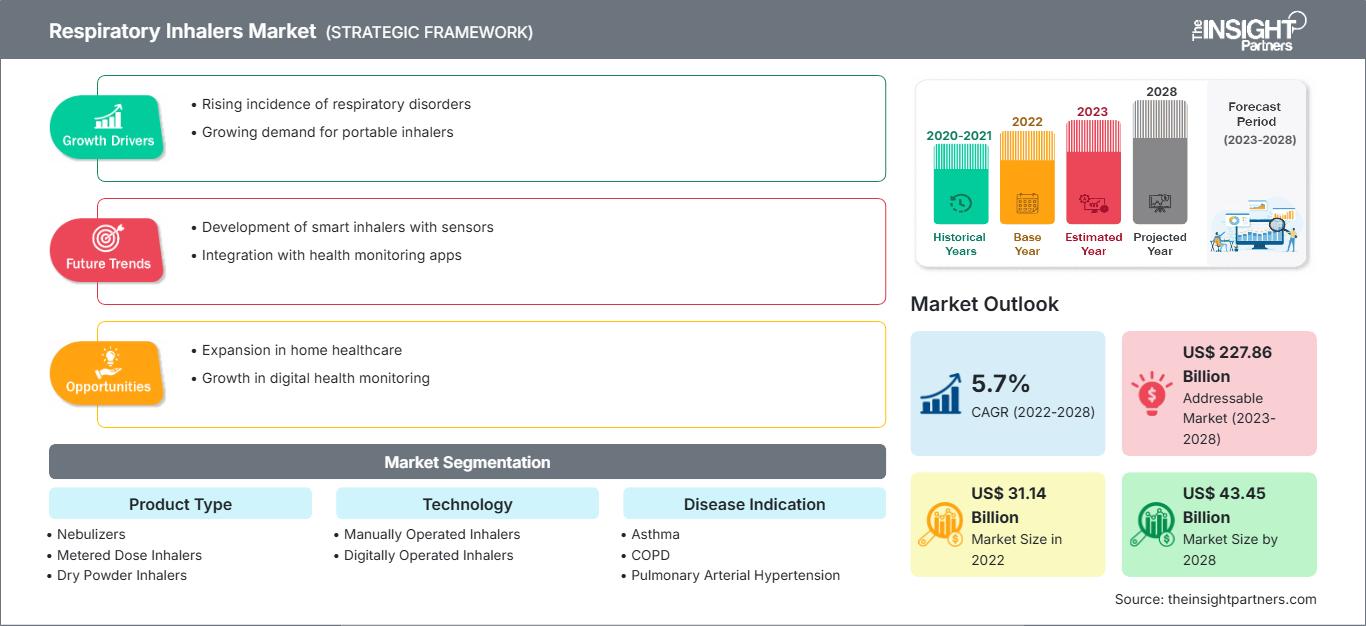
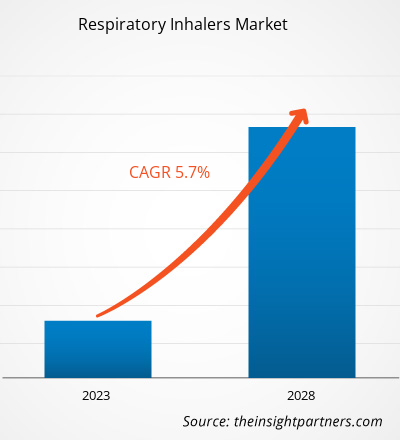
Si prevede che il mercato degli inalatori respiratori crescerà da 31.144,56 milioni di dollari nel 2022 a 43.446,19 milioni di dollari entro il 2028; si stima che registrerà un CAGR del 5,7% dal 2022 al 2028.
Gli inalatori respiratori vengono utilizzati per inalare farmaci essenziali per il trattamento delle malattie polmonari croniche. Sono utilizzati sia per prevenire che per trattare le riacutizzazioni di queste malattie croniche. Offrono il vantaggio di evitare l'esposizione sistemica al farmaco, garantendo al contempo che raggiungano direttamente i polmoni. Numerosi prodotti erogano i farmaci direttamente nelle vie aeree, come gli inalatori a polvere secca, gli inalatori predosati (MDI), i nebulizzatori e gli inalatori a nebbia leggera. Si prevede che il mercato degli inalatori respiratori crescerà a causa della crescente prevalenza di malattie respiratorie come l'asma, la broncopneumopatia cronica ostruttiva (BPCO), la fibrosi cistica, la sindrome da sovrapposizione asma-BPCO e altre.
Personalizza questo rapporto in base alle tue esigenze
Potrai personalizzare gratuitamente qualsiasi rapporto, comprese parti di questo rapporto, o analisi a livello di paese, pacchetto dati Excel, oltre a usufruire di grandi offerte e sconti per start-up e università
Mercato degli inalatori respiratori: Approfondimenti strategici
-
Ottieni le principali tendenze chiave del mercato di questo rapporto.Questo campione GRATUITO includerà l'analisi dei dati, che vanno dalle tendenze di mercato alle stime e alle previsioni.
Approfondimenti sul mercato degli inalatori respiratori
La crescente adozione di inalatori generici stimola la crescita del mercato degli inalatori respiratori
I farmaci per inalazione che utilizzano un'ampia gamma di dispositivi inalatori sono il cardine del trattamento farmacologico dell'asma e della BPCO. I pazienti affetti da asma e BPCO spesso necessitano di uno o più inalatori al giorno per mantenere sane le vie aeree. Pertanto, gli operatori sanitari sono preoccupati per il passaggio da un farmaco all'altro, in quanto può influire negativamente sul controllo della malattia a causa della scarsa aderenza terapeutica e dell'uso improprio dei dispositivi di somministrazione per aerosol. Con la crescente pressione sui bilanci sanitari e la contemporanea scadenza dei brevetti sui trattamenti inalatori consolidati, diversi prodotti sostitutivi generici vengono prodotti e forniti in tutto il mondo. Diversi inalanti utilizzati per il trattamento dell'asma includono formulazioni generiche, come albuterolo, levalbuterolo, ipratropio, budesonide e fluticasone/salmeterolo. Gli inalatori generici sono bioequivalenti alle versioni di marca e hanno lo stesso effetto sull'organismo. Il numero di inalatori generici per l'asma continua ad aumentare con la scadenza dei brevetti. Il 3 marzo 2020, la Food and Drug Administration (FDA) ha autorizzato gli inalatori generici per l'asma e la polvere generica di corticosteroidi inalatori. Nell'aprile 2020, la FDA ha approvato un inalatore generico, a base di solfato di albuterolo, per soddisfare le esigenze dei pazienti affetti da COVID-19 con difficoltà respiratorie. Con l'ingresso sul mercato di un numero maggiore di farmaci generici, si prevede una maggiore concorrenza, che a sua volta si traduce in una riduzione dei costi dei farmaci. Le riduzioni dei costi possono derivare anche dal lancio di un "farmaco generico approvato". Pertanto, la crescente adozione di inalatori generici e farmaci per l'asma sta migliorando l'aderenza alla terapia, trainando così il mercato degli inalatori respiratori.
Approfondimenti basati sulla tipologia di prodotto
In base alla tipologia di prodotto, il mercato degli inalatori respiratori è segmentato in nebulizzatori, inalatori dosati e inalatori a polvere secca. Il segmento degli inalatori a polvere secca è ulteriormente suddiviso in inalatori multidose a polvere secca e inalatori monodose a polvere secca. Il segmento degli inalatori dosati (MDI) è ulteriormente suddiviso in inalatori dosati pressurizzati e inalatori dosati connessi. Il segmento dei nebulizzatori è ulteriormente suddiviso in nebulizzatori ad aria compressa, nebulizzatori ad aria mesh e nebulizzatori ad aria ultrasonici. Nel 2022, il segmento degli inalatori a polvere secca ha rappresentato la quota di mercato maggiore. Tuttavia, si prevede che il segmento degli inalatori a dose misurata (MDI) registrerà il CAGR più elevato durante il periodo di previsione.
Approfondimenti basati sulla tecnologia
In base alla tecnologia, il mercato degli inalatori respiratori si divide in inalatori manuali e inalatori digitali. Il segmento degli inalatori manuali ha detenuto una quota di mercato maggiore nel 2022 e si prevede che registrerà un CAGR più elevato durante il periodo di previsione.
Approfondimenti regionali sul mercato degli inalatori respiratori
Le tendenze regionali e i fattori che influenzano il mercato degli inalatori respiratori durante il periodo di previsione sono stati ampiamente spiegati dagli analisti di The Insight Partners. Questa sezione illustra anche i segmenti e la distribuzione geografica del mercato degli inalatori respiratori in Nord America, Europa, Asia-Pacifico, Medio Oriente e Africa, America Meridionale e Centrale.
Ambito del rapporto di mercato sugli inalatori respiratori
| Attributo del rapporto | Dettagli |
|---|---|
| Dimensioni del mercato in 2022 | US$ 31.14 Billion |
| Dimensioni del mercato per 2028 | US$ 43.45 Billion |
| CAGR globale (2022 - 2028) | 5.7% |
| Dati storici | 2020-2021 |
| Periodo di previsione | 2023-2028 |
| Segmenti coperti |
By Tipo di prodotto
|
| Regioni e paesi coperti |
Nord America
|
| Leader di mercato e profili aziendali chiave |
|
Densità degli operatori del mercato degli inalatori respiratori: comprendere il suo impatto sulle dinamiche aziendali
Il mercato degli inalatori respiratori è in rapida crescita, trainato dalla crescente domanda da parte degli utenti finali, dovuta a fattori quali l'evoluzione delle preferenze dei consumatori, i progressi tecnologici e una maggiore consapevolezza dei benefici del prodotto. Con l'aumento della domanda, le aziende stanno ampliando la propria offerta, innovando per soddisfare le esigenze dei consumatori e sfruttando le tendenze emergenti, alimentando ulteriormente la crescita del mercato.
- Ottieni il Mercato degli inalatori respiratori Panoramica dei principali attori chiave
Approfondimenti basati sulle indicazioni della malattia
In base alle indicazioni della malattia, il mercato globale degli inalatori respiratori è segmentato in asma, BPCO, ipertensione arteriosa polmonare e altre patologie. Il segmento dell'asma ha detenuto la quota maggiore del mercato nel 2022 e si prevede che crescerà al CAGR più elevato durante il periodo di previsione.
Strategie inorganiche e organiche come fusioni e acquisizioni sono ampiamente adottate dalle aziende nel mercato degli inalatori respiratori. Di seguito sono elencati alcuni recenti sviluppi chiave del mercato:
- A settembre 2022, Beximco Pharmaceuticals ha lanciato ONRIVA TRIO BEXICAP, una capsula per inalazione a secco. ONRIVA TRIO BEXICAP è un preparato a base di indacaterolo (150 µg), glicopirronio (50 µg) e mometasone (160 µg). Agisce in 3 modi per controllare i sintomi dell'asma. Indacaterolo e glicopirronio aiutano i muscoli delle vie aeree polmonari a rimanere rilassati per prevenire la broncocostrizione, mentre il mometasone contribuisce a ridurre l'infiammazione. ONRIVA TRIO BEXICAP è indicato come terapia di mantenimento nell'asma persistente grave.
- A giugno 2021, Cipla ha annunciato di aver ricevuto l'approvazione definitiva per la sua Abbreviated New Drug Application (ANDA) per la soluzione inalatoria di arformoterolo tartrato 15 mcg/2 mL dalla Food and Drug Administration statunitense. La soluzione per inalazione di Arformoterol Tartrate da 15 mcg/2 mL di Cipla è una versione equivalente terapeutica generica classificata AN di Brovana di Sunovion Pharmaceuticals Inc.
- Nel febbraio 2022, AstraZeneca e Honeywell hanno annunciato i loro piani di collaborazione per lo sviluppo di inalatori respiratori di nuova generazione che utilizzano il propellente HFO-1234ze, che ha un potenziale di riscaldamento globale (GWP) inferiore fino al 99,9% rispetto ai propellenti attualmente utilizzati nei farmaci respiratori.
- Nel gennaio 2023, la FDA ha approvato Airsupra [inalatore pressurizzato a dose misurata (pMDI)] negli Stati Uniti per il trattamento al bisogno o la prevenzione della broncocostrizione. Airsupra è un farmaco di soccorso a combinazione fissa pressurizzato inalatore a dose misurata (pMDI), primo nella sua categoria, contenente albuterolo, un beta2-agonista a breve durata d'azione (SABA), e budesonide, un corticosteroide inalatorio antinfiammatorio (ICS) negli Stati Uniti.
Profili aziendali
- AstraZeneca Plc
- Beximco Pharmaceuticals Ltd.
- Boehringer Ingelheim International GmbH
- Cipla Ltd.
- GSK Plc
- Koninklijke Philips NV
- OMRON Corp
- PARI Respiratory Equipment, Inc.
- Teva Pharmaceutical Industries Ltd.
- OPKO Health, Inc.
- Analisi storica (2 anni), anno base, previsione (7 anni) con CAGR
- Analisi PEST e SWOT
- Valore/volume delle dimensioni del mercato - Globale, Regionale, Nazionale
- Industria e panorama competitivo
- Set di dati Excel
Report recenti
Testimonianze
Motivo dell'acquisto
- Processo decisionale informato
- Comprensione delle dinamiche di mercato
- Analisi competitiva
- Analisi dei clienti
- Previsioni di mercato
- Mitigazione del rischio
- Pianificazione strategica
- Giustificazione degli investimenti
- Identificazione dei mercati emergenti
- Miglioramento delle strategie di marketing
- Aumento dell'efficienza operativa
- Allineamento alle tendenze normative






















 Ottieni un campione gratuito per - Mercato degli inalatori respiratori
Ottieni un campione gratuito per - Mercato degli inalatori respiratori