The Asia Pacific anti-viral therapies market is expected to reach US$ 16,118.52 million by 2027 from US$ 8,135.93 million in 2019; it is estimated to grow at a CAGR of 9.0% from 2020 to 2027.
Increasing R&D expenditures in pharmaceutical companies and rising government support for research activities and clinical trials are the key factors driving the growth of the Asia Pacific Anti-Viral Therapies market in this region. However, high cost of drug development is a key factor hindering the market growth in Asia Pacific.
Antiviral therapy is one of the most exciting branches of virology. These therapies are based on several strategies—direct-acting antivirals target viral proteins, enzymes, or nucleic acids; passive antibodies neutralize circulating viruses; and several other antivirals target cellular proteins or processes essential for viral replication.
Asia is emerging as a powerhouse of pharmaceutical R&D on the back of vast patient population, quality data, lower costs, and skilled manpower availability. For instance, AstraZeneca has announced its plans to spend US$ 100 million in expanding its research operations in China, with the main focus on a program to better understand Chinese patients’ genetic profile to develop new therapies for the Chinese population. According to experts, such costs are reduced to half when clinical research activities are outsourced from low-cost economies such as India. Other Asian countries such as Malaysia, South Korea, and Taiwan are also attracting a number of international pharma companies to outsource their R&D activities. The government of Taiwan is planning to spend US$ 4.48 billion on biomedical research. Regulatory environments in these countries are conducive to clinical trials. Vast patient population, low procedure costs, huge R&D workforce, and favorable regulatory environment are the main factors driving the transformation of Asia into the hub of R&D activities.
Countries in Asia Pacific are experiencing the rise in number of COVID-19 cases. A research team from Beijing, China has successfully tested a drug on animal that would reduce the recovery time for COVID-19 patients and confer short-term immunity to the virus. Further, the National Medical Products Administration of China has approved the anti-viral drug Favilavir to treat COVID-19 patients. Additionally, China has approved to assess a third vaccine candidate against COVID-19.
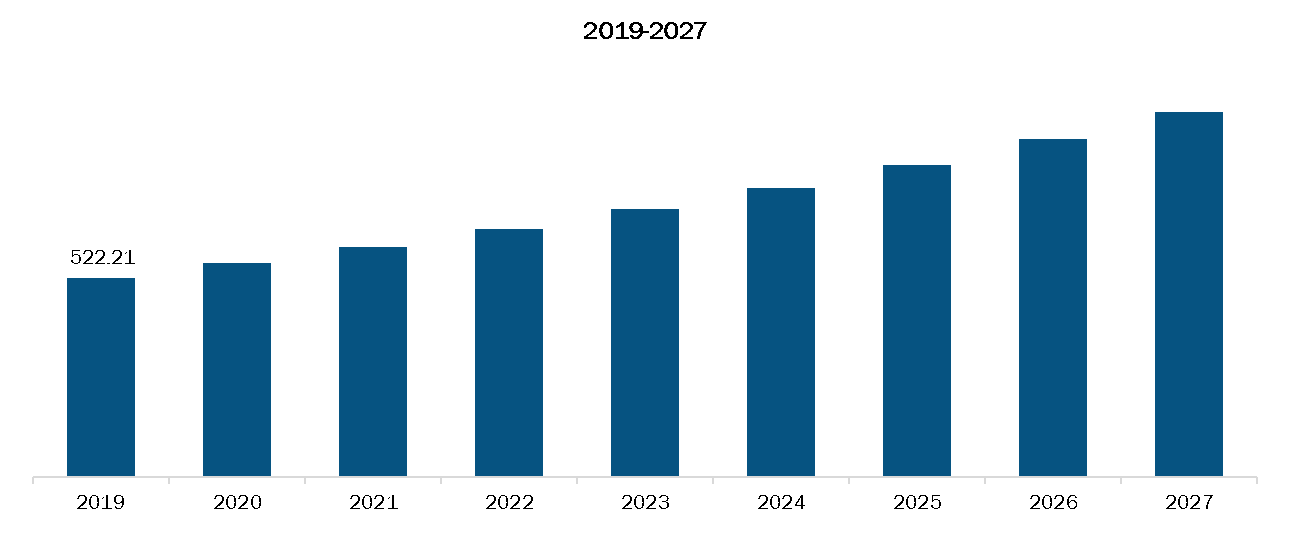
Rest of Asia Pacific Anti-Viral Therapies Market, Revenue and Forecast to 2027 (US$ Million)
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
ASIA PACIFIC ANTI-VIRAL THERAPIES MARKET SEGMENTATION
By Type
- Branded Drugs
- Generic Drugs
By Mechanism of Action
- Nucleotide Polymerase Inhibitors
- Reverse Transcriptase Inhibitors
- Protease Inhibitors
- Others
By Application
- HIV
- Hepatitis
- Virus Influenza
- Herpes
- Others
By Country
- China
- Japan
- India
- South Korea
- Australia
Company Profiles
- Aurobindo Pharma Ltd
- AbbVie Inc
- GlaxoSmithKline plc
- AstraZeneca
- F. HOFFMANN-LA ROCHE LTD
Asia Pacific Anti-Viral Therapies Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 8,135.93 Million |
| Market Size by 2027 | US$ 16,118.52 Million |
| CAGR (2020 - 2027) | 9.0% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
Asia-Pacific
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For