Disclosing Tablets Market Developments, Trends, Opportunities, and Forecast by 2031
Disclosing Tablets Market Size and Forecasts (2021 - 2031), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Composition(Iodine, Fluorescein, and Erythrosine); Age Group(children, adults, and geriatric population); Distribution Channel (online (e-commerce) and medical pharmacies) , and Geography (North America, Europe, Asia Pacific, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Mar 2026
- Report Code : TIPRE00025964
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
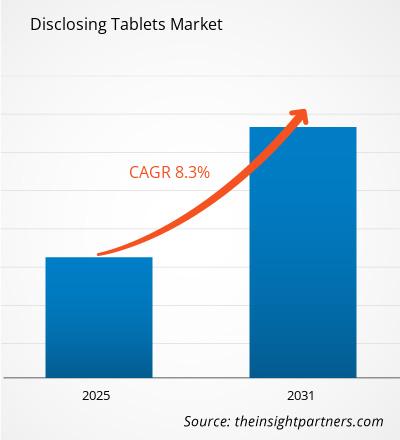
The Disclosing Tablets Market is expected to register a CAGR of 8.30% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report presents an analysis based on by age group (pediatrics, adults, and elderly). The report is segmented by application (orthopedic physical therapy, geriatric physical therapy, neurological physical therapy, cardiopulmonary and pulmonary physical therapy, and others). The report further provides analysis based on end use (hospitals, private practices, outpatient clinics, sports and fitness facility centers, and others). The global analysis is further broken-down at regional level and major countries. The market size and forecast at global, regional, and country levels for all the key market segments are covered under the scope. The report offers the value in USD for the above analysis and segments. The report provides key statistics on the market status of the key market players and offers market trends and opportunities.
Purpose of the Report
The report Disclosing Tablets Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Disclosing Tablets Market Segmentation
Composition
- Iodine
- Fluorescein
- Erythrosine
Age Group
- children
- adults
- geriatric population
Distribution Channel
- online
- medical pharmacies
Geography
- North America
- Europe
- Asia-Pacific
- South and Central America
- Middle East and Africa
Customizee This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONDisclosing Tablets Market: Strategic Insights

-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Disclosing Tablets Market Growth Drivers
- Oral Hygiene Expands Awareness: The growing concern over oral hygiene is incensed demands from consumers that will be converted into the use of disclosing tablets for accessing certain areas of the patients' mouths with regards to hands-on education on which areas an individual needs special attention, and hence deserving mention at both ends-personal dental care as well as professional dental care. Such health campaigns have publicity effort going on in the practicing dental professionals, which intends to promote it in the public interest of the consumer to improve oral health behaviors.
- Growth Forecasts in Preventative Dental Care: As preventive health care becomes increasingly integrated into the mainstream, the importance of disclosing tablets has grown in professional practices and household use. Previously, disclosing tablets were used more for professional practice only; now they would be able to find their place in an individual's home due to use of daily practice for oral hygiene, therefore ensuring that there exists a greater opportunity for demand for such items.
- Increase in Pediatric Dental Care: Due to the localization of early education as a priority for many nations, there is indeed more focus on pediatric oral health. Increased use of disclosing tablets in schools and from parents is to enable children to see the areas where plaque builds up and subsequently teach them how to brush correctly; thus, such will broaden the application of these tablets in the pediatric field.
Disclosing Tablets Market Future Trends
- Integration with Intelligent Oral Care Appliances: With the coming of smart toothbrush and connected oral care devices, it is most likely that some forms of disclosing tablets will be integrated with these technologies. These gizmos could record platforms with brushing habits and the efficiency of plaque removal, whereas with disclosing tablets, users will be able to visualize areas that still need attention combining of conventional and modern technology in oral care enhancement.
- Eco-Friendly and Always Natural Ingredients:Product demand is now into green or sustainable products, and most manufacturers for that reason concentrate on developing natural and biodegradable disclosing tablets. That is the reason why consumers typically select products showing a lesser impact on the environment, thus compelling companies to develop plant-based or chemical-free alternatives that would still get the job done but with lesser ruthless ingredients to both users and the environment.
- Growing Utilization in Professional Dental Clinics: The use of disclosing tablets is becoming an integral part of dental clinics, beyond general checkups; specialized therapy includes disclosing tablets. Among the now numerous dental practitioners using these tablets as part of preventive care programs and education for patients are accounts of being valuable tools or resources for these dentists to show the effectiveness of patients' oral hygiene practices or areas on which the patient needs improvement.
Disclosing Tablets Market Opportunities
- Development into Emerging Markets: Emerging markets, particularly those in Asia and Africa, provide excellent business development opportunities because access to dental care has increased and a health-conscious public is on the rise. Manufacturers may give place to those markets by providing reasonably priced disclosing tablet products carved from preventive dates of oral care and educating consumers as well as dental professionals on the benefits of their usage.
- Partnerships with Brands for Dental Products:Cooperation with established oral care brands, such as manufacturers of toothpaste and toothbrushes, would yield the opportunity for cross-promotions by putting disclosing tablets as additions to packaged collaterals or as simply value-added products for their customers.
- Developing Specialized Disclosing Tablets for Sensitive Teeth: Many people who have sensitive teeth or gums find it difficult to employ conventional plaque detection; thus, special disclosing tablets designed for sensitivity along with the soothing agents and gentle dyes are an interesting application for a growing but niche market that needs some gentle products for oral care.
Disclosing Tablets Market Regional Insights
The regional trends and factors influencing the Disclosing Tablets Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Disclosing Tablets Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Disclosing Tablets Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 8.30% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Composition
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Disclosing Tablets Market Players Density: Understanding Its Impact on Business Dynamics
The Disclosing Tablets Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Disclosing Tablets Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Disclosing Tablets Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Disclosing Tablets Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
/
country-wise market wherein 2021-2022 are the historic years, 2023 is considered to be the base year, and the forecast will be provided till 2031, along with CAGR (%)
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For