ePRO, ePatient Diaries, and eCOA Market Overview and Growth Trends (2026-2034)
ePRO, ePatient Diaries, and eCOA Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type of Solution (ECOA (Electronic Clinical Outcome Assessments), EPROs (Patient Reported Outcomes), ClinROs (Clinician Reported Outcomes), ObsROs (Observer Reported Outcomes), PerfOs (Performance Outcomes), and ePatient Diaries); Modality (Computer, and Mobile Devices (Smartphones and Tablets)); End User (Clinical Trial Sponsors, Contract Research Organizations (CRO's), Hospitals, Academic Institutes, Pharmaceutical Companies, and Others), and Geography
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Mar 2026
- Report Code : TIPRE00018488
- Category : Technology, Media and Telecommunications
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
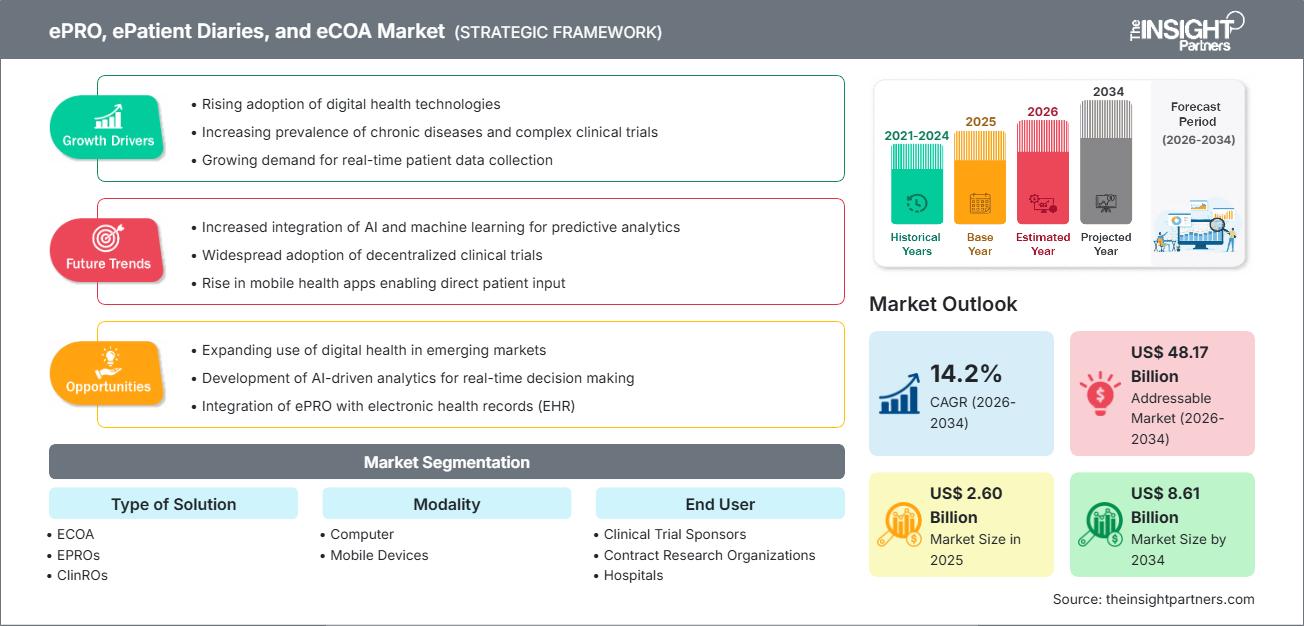
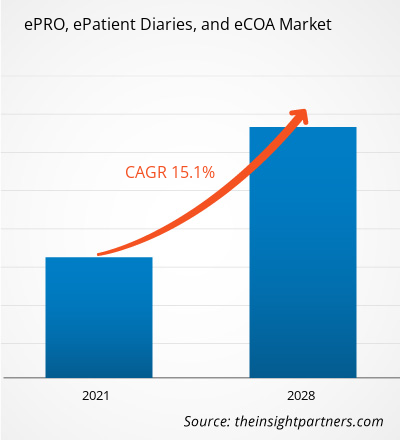
The ePRO, ePatient Diaries, and eCOA market was valued at US$ 2.60 billion in 2025. It is expected to reach US$ 8.61 billion by 2034, registering a CAGR of 14.2% during 2026–2034.
ePRO, ePatient Diaries, and eCOA Market Analysis
The forecast for the ePRO, ePatient Diaries, and eCOA market is very strong due to several factors:
- This trend toward virtual and decentralized clinical trials, accelerated in particular by the COVID-19 pandemic, is driving the adoption of electronic assessment tools.
- Increasing demand for real-time, high-quality patient data: eCOA platforms can capture patient-reported outcomes, clinician-reported, observer-reported, and performance outcomes electronically, improving data integrity and timeliness.
- Reduction in administrative burden and cost for sponsors and CROs: ePRO/eCOA systems streamline data collection, minimize data entry errors, and accelerate trial timelines.
- Regulatory and industry push: More clinical trials are adopting patient-centric digital platforms, and there is growing regulatory comfort with electronic outcome assessments.
ePRO, ePatient Diaries, and eCOA Market Overview
Electronic Clinical Outcome Assessments (eCOA), ePROs (electronic Patient Reported Outcomes), and ePatient Diaries together form a critical infrastructure for modern clinical trials. These solutions allow trial participants (patients, clinicians, observers) to report outcomes digitally using devices such as computers, tablets, or smartphones.
These systems enhance the data quality, compliance, and efficiency of trials: they reduce recall bias, timestamp entries, and permit centralized monitoring. For sponsors and CROs, such platforms reduce cost, lower discrepancies, and support patient-centric trial designs, all of which contribute to faster, more reliable studies.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONePRO, ePatient Diaries, and eCOA Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
ePRO, ePatient Diaries, and eCOA Market Drivers & Opportunities
Market Drivers
- Rise in Clinical Trials: The number of global clinical trials continues to increase, boosting demand for efficient digital outcome reporting.
- Patient Centricity: The clinical trials industry is increasingly focused on patient experience, making ePRO and ePatient Diaries more attractive.
- Regulatory & Technological Enablers: Widespread smartphone adoption and the increased acceptance of electronic assessments by regulatory authorities each encourage the deployment of eCOA.
Market Opportunities
- Mobile Device Penetration: The shift to using mobile devices-smartphones or tablets-to conduct assessments opens up scalable, patient-friendly modalities.
- Broader Adoption by CROs & Sponsors: As sponsors and CROs move toward digital-first trials, there's a growing opportunity to deploy integrated eCOA/ePRO platforms.
- Integration with EHR / Real-World Data: There is potential for eCOA tools to integrate with electronic health records (EHRs) and real-world evidence systems, strengthening data-rich, patient-centered research.
ePRO, ePatient Diaries, and eCOA Market Report Segmentation Analysis
By Type of Solution:
- eCOA
- ePROs
- ClinROs
- ObsROs
- PerfOs
- ePatient Diaries
By Modality:
- Computer
- Mobile Devices
By End-User:
- Clinical Trial Sponsors
- Contract Research Organizations (CROs)
- Hospitals
- Academic Institutes
- Pharmaceutical Companies
By Geography:
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- South & Central America
The regional trends and factors influencing the ePRO, ePatient Diaries, and eCOA Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses ePRO, ePatient Diaries, and eCOA Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
ePRO, ePatient Diaries, and eCOA Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 2.60 Billion |
| Market Size by 2034 | US$ 8.61 Billion |
| Global CAGR (2026 - 2034) | 14.2% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Type of Solution
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
ePRO, ePatient Diaries, and eCOA Market Players Density: Understanding Its Impact on Business Dynamics
The ePRO, ePatient Diaries, and eCOA Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the ePRO, ePatient Diaries, and eCOA Market top key players overview
ePRO, ePatient Diaries, and eCOA Market Regional Share Analysis
- North America: Probably has the largest share, considering a high volume of clinical trials, a robust regulatory framework, and the most advanced digital infrastructure..
- Europe: Adoption driven by pharmaceutical innovation, strong CRO presence, and regulatory support for decentralized trials.
- Asia Pacific: Emerging region with increasing research activity, rising clinical trials, and growing digital health adoption.
- South & Central America, Middle East & Africa: Growing opportunities in decentralized trials, but adoption may be limited by infrastructure and regulatory factors.
ePRO, ePatient Diaries, and eCOA Market Players Density & Competitive Landscape
Competitive Differentiation Factors:
- Seamless integration with EDC (Electronic Data Capture) and EHR systems.
- Advanced modalities: mobile, BYOD (Bring Your Own Device) capabilities.
- Patient engagement features: reminders, a user‑friendly interface, and timestamping.
- Regulatory compliance and data security practices.
- Analytics capabilities: real‑time dashboards, data cleaning, and trend detection.
Strategic Moves & Opportunities:
- Collaborations between CROs and eCOA vendors to embed assessment tools into trial protocols.
- Mergers and acquisitions to combine platform capabilities (e.g., ePRO + eCOA + eConsent).
- Development of AI/ML analytics for patient-reported data, predictive modeling, and compliance risk.
- Expansion in emerging geographies (APAC, Latin America) through partnerships.
ePRO, ePatient Diaries, and eCOA Market Major Companies
- Bracket Global LLC (Signant Health)
- ERT Clinical
- ArisGlobal LLC
- ICON Plc
- PAREXEL International
- Kayentis
- Anju Software, Inc.
- The Diary Pte. Ltd
- Dassault Systèmes SE
Other companies analyzed during the course of research:
- YPrime, LLC
- Veeva Systems Inc.
- Clario (formerly ERT + Bioclinica)
- IQVIA Holdings Inc.
- CRF Health (merged into Signant Health but still evaluated historically)
- DataMeds Solutions
- Quest Diagnostics (eClinical Solutions division)
- Medable, Inc.
ePRO, ePatient Diaries, and eCOA Market News & Recent Developments
- Veeva Systems launched MyVeeva for Patients, a comprehensive platform enabling ePRO, eCOA, eConsent, and remote patient visits, making it easier to run patient-centric, paperless trials.
- YPrime, LLC announced the launch of a next-generation eCOA platform in January 2021, improving user experience, site satisfaction, and data cleanliness.
- Growing regulatory and patient-centric trends: Studies show increasing CRO and sponsor interest in real‑world patient data, including ePRO/eCOA data, for decision-making.
ePRO, ePatient Diaries, and eCOA Market Report Coverage & Deliverables
The Insight Partners’ ePRO, ePatient Diaries, and eCOA Market Forecast (2021-2034) report includes:
- Market size & forecast (global, regional) for 2021–2034
- Market dynamics: drivers, restraints, opportunities, and trends
- Segmentation analysis by type of solution, modality, end-user, and geography
- Strategic insights & key market trends
- Competitive landscape: profiles of major players, market concentration, differentiators
- Recent developments and innovations
Frequently Asked Questions
Ankita is a dynamic market research and consulting professional with over 8 years of experience across the technology, media, ICT, and electronics & semiconductor sectors. She has successfully led and delivered 100+ consulting and research assignments for global clients such as Microsoft, Oracle, NEC Corporation, SAP, KPMG, and Expeditors International. Her core competencies include market assessment, data analysis, forecasting, strategy formulation, competitive intelligence, and report writing.
Ankita is adept at handling complete project cycles—from pre-sales proposal design and client discussions to post-sales delivery of actionable insights. She is skilled in managing cross-functional teams, structuring complex research modules, and aligning solutions with client-specific business goals. Her excellent communication, leadership, and presentation abilities have enabled her to consistently deliver value-driven outcomes in fast-paced and evolving market environments.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For