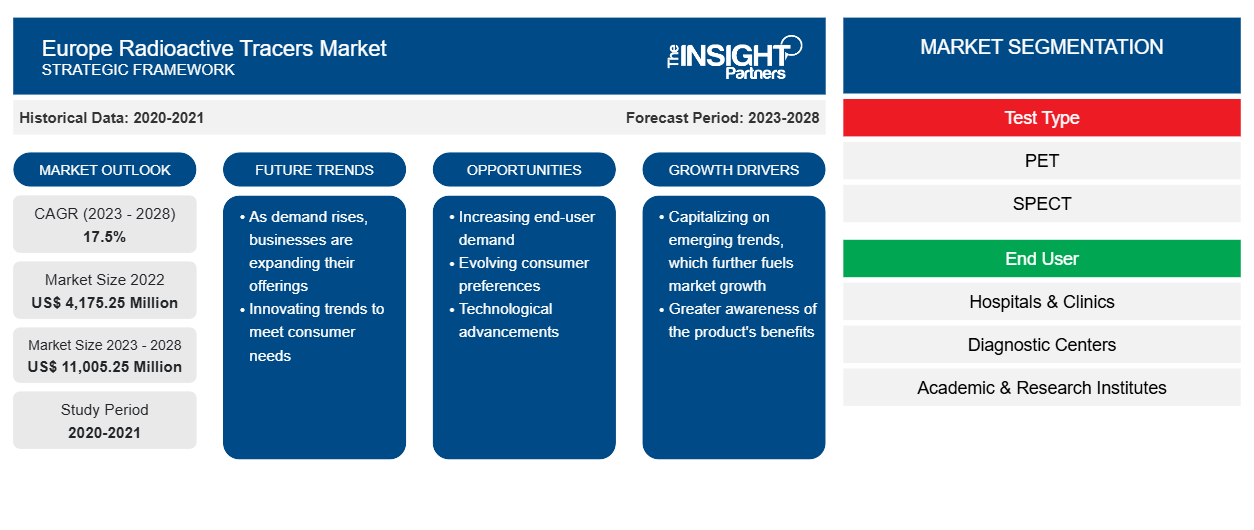
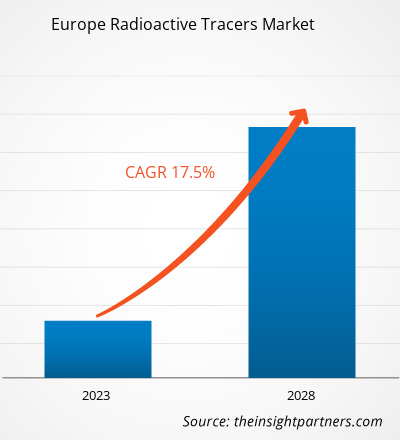
The Europe radioactive tracers market size is expected to grow from US$ 4,175.25 million in 2022 to US$ 11,005.25 million by 2028; it is estimated to record a CAGR of 17.5% during 2023–2028.
A radioactive tracer is a chemical compound in which one or more atoms are replaced by a radioisotope. Radiotracers can be used to study chemical reactions based on the monitoring of their radioactive decay. They are also used to visualize flow in techniques such as single photon emission computed tomography (SPECT), positron emission tomography (PET), and computed radioactive particle tracking (CARPT).
The Europe radioactive tracer market is segmented on the basis of tracer type, end user, test type, application, and geography. Based on country, the market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. The report offers insights and in-depth analysis of the Europe radioactive tracers market, emphasizing on parameters such as market trends, technological advancements, and market dynamics, along with the competitive landscape analysis of the world's leading market players.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Europe Radioactive Tracers Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Europe Radioactive Tracers Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
New Initiatives for Radioactive Tracer R&D to Offer Opportunities for Europe Radioactive Tracers Market Growth During Forecast Period
The European Union Committee has taken various initiatives for boosting research & development activities in the field of radioactive tracers and radioisotopes. PRISMAP is a European medical radionuclide program that was started to provide access to novel radioisotopes of high purity grade for medical research. According to data provided under this program, out of the ~3,000 different radioisotopes synthesized by scientists in laboratories under European Union, only a handful are used regularly for medical procedures, mostly for imaging. Therefore, to increase the innovation and approval of these radioisotopes, the European Commission is planning to launch European Radioisotope Valley Initiative (ERVI) to maintain the region’s global leadership in the supply of medical radioisotopes and to help accelerate the development and introduction of new radioisotopes along with their production methods. Such a rise in the number of initiatives for boosting the supply of radioactive tracers for research purposes is expected to fuel the growth of the radioactive tracers market during the forecast period.
Many hospitals and research institutes focus on developing and using different radioactive tracers for the diagnosis of chronic diseases. Massachusetts General Hospital (MGH) is focused on using a new PET tracer [18F]3F4AP for the diagnosis of multiple sclerosis, Alzheimer’s disease, mild cognitive impairment, and traumatic brain injury. According to the National Library of Medicine (ClinicalTrials.gov), the use of [18F]3F4AP in the diagnosis of multiple sclerosis is in Phase 1 clinical trial, whereas the use of [18F]3F4AP for the diagnosis of Alzheimer’s disease, mild cognitive impairment, and traumatic brain injury is also in the Phase 1 trial. The ongoing research for the discovery of new radioactive tracers for the diagnosis of neurological disorders is anticipated to fuel the growth of the radioactive tracers market in the coming years.
Europe Radioactive Tracers Market-Test Type Insights
The Europe radioactive tracers market, based on test type, is segmented into PET, SPECT, and other test types. In 2022, the PET segment held the largest market share. The market for the same segment is expected to grow at the fastest CAGR in the coming years. PET provides quantitative information on the distribution of positron emitter-labeled radiopharmaceuticals (PET radiopharmaceuticals) in the body. PET imaging procedure involves the use of several expensive equipment, such as a cyclotron for radionuclide production, automated chemistry devices, purification instrumentation, and PET cameras. The advantages of PET over traditional radionuclide imaging techniques include higher spatial resolution and sensitivity, quantification of activity, and synthesizing physiologically useful tracers. PET uses small amounts of radioactive materials termed radiotracers, requiring a special camera and a computer to help evaluate the functioning of specific organs and tissues. By identifying bodily changes at the cellular level, PET may detect the early signs of disease before it is evident on other imaging tests. Nowadays, nearly all PET scans are performed in combination with CT scanners. The combined PET/CT scans provide images that locate the anatomic location of abnormal metabolic activity inside the body. The combined scans provide more accurate diagnoses than the two scans performed separately. Although the PET segment is expected to witness significant growth during the forecast period owing to several advantages, the high cost of PET scanners hinders its adoption in scanning diseases. Therefore, the Europe radioactive tracers market for the PET segment is expected to experience significant growth during the forecast period.
Europe Radioactive Tracers Market, by Test Type – 2022 and 2028
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Impact of COVID-19 on Europe Radioactive Tracers Market
The COVID-19 pandemic has led to profound changes in hospital operations, including nuclear medicine (NM) practice across Europe. A literature search on PubMed covered COVID-19 studies published up until January 2021. As per the findings, the pandemic posed significant challenges for NM departments, and a reduction in the workforce was experienced in every center in Europe in 2020. NM departments introduced restriction measures to limit COVID-19 transmission, including rescheduling non-high-priority procedures. Also, some of the departments experienced delays in radioactive tracers supply or technical assistance amid this crisis. As a result, the pandemic resulted in a significant reduction in diagnostic NM procedures and a reduced level of care for patients affected by diseases other than COVID-19, such as cancer or acute cardiovascular disease. According to the results of the British Nuclear Medicine Society’s (BNMS) COVID-19 survey, ~97% of NM departments introduced procedures to limit SARS-CoV-2 transmission; at 68% of sites, standardized operating procedures were developed for running departments in pandemic situations.
However, new findings supporting innovations in the methods for COVID-19 diagnosis are expected to accelerate the growth of the radioactive tracers market in Europe. As per the research study by Chentao Jin et al., published in the European Journal of Nuclear Medicine and Molecular Imaging in May 2021, PET is expected to offer pathophysiological alternations of COVID-19 and facilitate the clinical management of patients.
Europe Radioactive Tracers Market – Market Segmentation
The Europe radioactive tracers market, based on test type, is segmented into PET, SPECT, and others. By end user, the market is segmented into hospitals & clinics, diagnostic centers, academic & research institutes, and others. The Europe radioactive tracers market, by tracer type, is segmented into Technetium-99m and Tc-97m, Iodine-13, Iron-59, Lutetium-171, Rubidium (Rb-82) Chloride and Ammonia (N-13), Scandium-46, Seaborgium-269, Hassium-269, Gallium Citrate Ga 67, Prostate-Specific Membrane Antigen (PSMA) (Ga-68), FDDNP (F-18) and FDOPA (F-18), Phosphorus-32 and chromium-51, Thallium-201, F-18 FDG, F-18 FAPI, Ga-68 FAPI, F-18 PSMA, DOTATOC/DOTANOC/DOTATATE (Ga-68), and others. The Europe radioactive tracers market, by application, is segmented into oncology, pulmonary, neurology, cardiology, and others. Based on country, the Europe radioactive tracers market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe.
Europe Radioactive Tracers Market – Company Profiles
- Rotem Industries Ltd.
- ABX Advanced Biochemical Compounds GmbH
- Invicro LLC.
- Cardinal Health
- Newcastle University
- Novartis
- Curium
- Blue Earth Diagnostics
- IBA Radiopharma Solutions
- General Electric Company
Europe Radioactive Tracers Market Regional Insights
The regional trends and factors influencing the Europe Radioactive Tracers Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Europe Radioactive Tracers Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Europe Radioactive Tracers Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 4,175.25 Million |
| Market Size by 2028 | US$ 11,005.25 Million |
| Global CAGR (2023 - 2028) | 17.5% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Test Type
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
Europe Radioactive Tracers Market Players Density: Understanding Its Impact on Business Dynamics
The Europe Radioactive Tracers Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Europe Radioactive Tracers Market top key players overview
Frequently Asked Questions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely - analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You'll receive access to the report within 4-6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we'll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we're happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you're considering, and we’ll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report’s scope, methodology, customization options, or which license suits you best, we're here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we’ll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we'll ensure the issue is resolved quickly so you can access your report without interruption.















The List of Companies - Europe Radioactive Tracer Market
- Rotem Industries Ltd
- ABX advanced biochemical compounds GmbH
- Invicro LLC
- Cardinal Health Inc
- Newcastle University
- Novartis AG
- Curium
- Blue Earth Diagnostics Limited
- General Electric Co
- IBA Radiopharma Solutions






 Get Free Sample For
Get Free Sample For