Fluorine-18 Market Size, Trends & Forecast 2031
Fluorine-18 Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (FDG, NaF, and Others), Application (Oncology, Cardiology, Neurology, and Others), End User (Hospitals, Diagnostic and Imaging Centers, and Others), and Geography (North America, Europe, Asia Pacific, South and Central America, and Middle East and Africa)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Oct 2025
- Report Code : TIPRE00038973
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 200
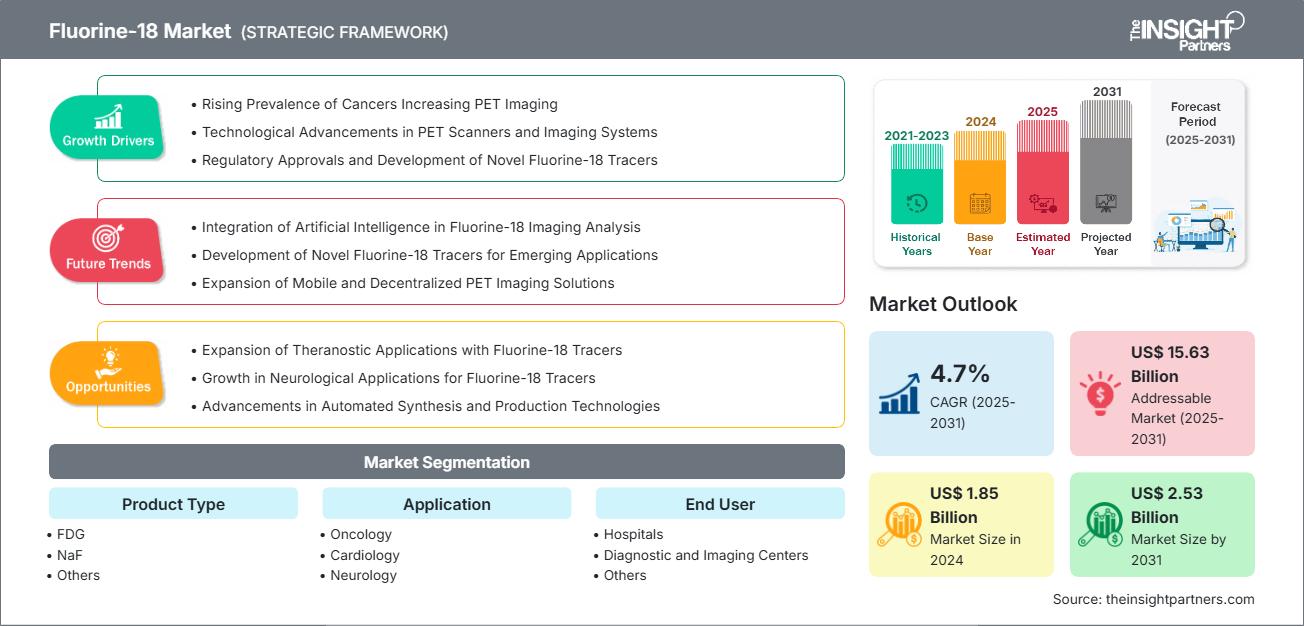
The fluorine-18 market size is projected to reach US$ 2.53 billion by 2031 from US$ 1.85 billion in 2024. The market is expected to register a CAGR of 4.7% during 2025–2031.
Fluorine-18 Market Analysis
The market's growth is driven by the rising prevalence of cancers, increasing pet imaging, technological advancements in PET scanners and imaging systems, and regulatory approvals and development of novel fluorine-18 tracers. Integration of artificial intelligence in fluorine-18 imaging analysis and development of novel fluorine-18 tracers contributes to market development. Expansion of theranostic applications with fluorine-18 tracers and growth in neurological applications for fluorine-18 tracers is expected to create ample opportunities for the fluorine-18 market growth.
Fluorine-18 Market Overview
The fluorine-18 market is driven by the increasing global incidence of cancer as well as advancement in the field of nuclear medicine. Besides the dominant role of Fluorodeoxyglucose (FDG), which accounts for more than 80% of the PET-CT scan market, the usage of PET in cardiology and the central nervous system is also increasing rapidly. Apart from that, GE HealthCare's introduction of Flyrcado (flurpiridaz F 18) in 2025 for myocardial perfusion imaging is expected to be a milestone in cardiovascular assessments, as the precision of the technique will be improved considerably. Hospitals are the leading end-users of the sector, which creates the highest volume of demand. The characteristic of the isotope, such as its limited life (110 minutes), has induced new installations of cyclotrons at the site. Due to the availability of cutting-edge technology in the region, North America is still the most significant geographical area for the market. However, Asia Pacific has become a high-potential market owing to the influx of healthcare investments and is the most likely area for market growth.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONFluorine-18 Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Fluorine-18 Market Drivers and Opportunities
Market Drivers:
- Rising Prevalence of Cancers Increasing PET Imaging: One of the main reasons for the rise in demand for Fluorine-18 radiotracers is the worldwide increase in cancer incidence, with a significant proportion of cancers being those tumors whose metabolic profiling by Fluorine-18-based positron emission tomography (PET) scans is possible.
- Technological Advancements in PET Scanners and Imaging Systems: The advances in positron emission tomography (PET) hardware and combined modalities have significantly increased the use of clinically relevant Fluorine-18 radiotracers, allowing the figures to surpass their previous limits as diagnostic tools.
- Regulatory Approvals and Development of Novel Fluorine-18 Tracers: One of the main factors which have boosted the market for Fluorine-18-labeled innovative tracers is the increasing number of regulatory approvals. Besides broadening the therapeutic windows, these tracers have been able to increase the specificity of the disease over a wide range of pathologies, which has made their clinical integration and production requirements to progress rapidly.
Market Opportunities:
- Expansion of Theranostic Applications with Fluorine-18 Tracers: Combining Fluorine-18 PET with theranostic systems can open the way for a major change in the Fluorine-18 market, that is, diagnostic accuracy as well as therapy monitoring can be made possible.
- Growth in Neurological Applications for Fluorine-18 Tracers : A major source of opportunity for the Fluorine-18 market is the increasing usage of the neuroimaging field, which comes from the escalating occurrence of nervous system diseases, amongst which Alzheimer’s disease and other dementias take the leading position.
- Advancements in Automated Synthesis and Production Technologies: One of the major changes that led to a significant step in simplifying the production of Fluorine-18 radiotracers is the invention of automated synthesis modules. These innovations provide a great chance for improving production efficacy, saving money, and opening new markets.
Fluorine-18 Market Report Segmentation Analysis
The fluorine-18 market is divided into different segments to give a clearer view of how it works, its growth potential, and the latest trends. Below is the standard segmentation approach used in most industry reports:
By Product Type:
- FDG: FDG (fluorodeoxyglucose) is the most important radiotracer for positron emission tomography (PET) imaging, basically due to its function to make visible the glucose metabolism in pathological tissues. The half-life of FDG is about 110 minutes, which makes it necessary to be produced by a cyclotron on-site, allowing a quick delivery to the imaging centers for use on the same day.
- NaF: Sodium Fluoride (NaF) is recognized as the most effective agent for localizing bone changes, particularly in metastatic bone disease, which is why it is the primary diagnostic tool in cancer diseases. Because the product has high bone affinity, it allows for the acquisition of accurate images of skeletal structures, thereby providing better sensitivity compared to conventional bone scintigraphy agents.
- Others: The Others encompasses several non-FDG radiotracers such as FLT (fluorothymidine), FMISO (fluoromisonidazole), and FET (fluoroethyltyrosine) that are leading to the development of new diagnostic areas that utilize PET imaging technology. The tracers are the main source for the rare indications of oncology, neurology, and hypoxia imaging fields.
By Application:
- Oncology
- Cardiology
- Neurology
- Others
By End-User Industry:
- Hospitals
- Diagnostic and Imaging Centers
- Others
Each end user in the Fluorine-18 market has distinct handling, safety, and regulatory needs, shaping equipment selection and operational protocols.
By Geography:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
The fluorine-18 market in Asia Pacific is expected to witness the fastest growth. The surging demand for positron emission tomography (PET) imaging, driven by rising cancer incidences and expanding healthcare infrastructure, is likely to drive the market.
Fluorine-18
Fluorine-18 Market Regional InsightsThe regional trends and factors influencing the Fluorine-18 Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Fluorine-18 Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Fluorine-18 Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 1.85 Billion |
| Market Size by 2031 | US$ 2.53 Billion |
| Global CAGR (2025 - 2031) | 4.7% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Fluorine-18 Market Players Density: Understanding Its Impact on Business Dynamics
The Fluorine-18 Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Fluorine-18 Market Share Analysis by Geography
Asia Pacific is expected to grow the fastest in the next few years. Emerging markets in Latin America, the Middle East, and Africa also have many untapped opportunities for fluorine-18 providers to expand.
The fluorine-18 market grows differently in each region owing to the increasing prevalence of oncology and other conditions, technological advancements in PET scanners and imaging systems, along with regulatory approvals and the development of F-18 tracers. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds a significant portion of the global market
-
Key Drivers:
- The rising demand for PET imaging in oncology, the increased prevalence of cancer, advancements in radiopharmaceuticals, and growing investments in nuclear medicine infrastructure
- Trends: Advancements in radiopharmaceutical production
2. Europe
- Market Share: Substantial share due to early adoption of Fluorine-18 for PET Imaging
-
Key Drivers:
- Increasing cancer incidence, growing preference for PET imaging, supportive government healthcare initiatives, and expanding use of radiopharmaceuticals in personalized and precision medicine.
- Trends: Increasing integration of Fluorine-18 in personalized medicine
3. Asia Pacific
- Market Share: Fastest-growing region with rising market share every year
-
Key Drivers:
- Rising healthcare expenditure, increasing awareness of early cancer diagnosis, expanding PET imaging facilities, and growing investment in nuclear medicine and radiopharmaceutical research.
- Trends: Growing partnerships between hospitals and radiopharmaceutical companies
4. South and Central America
- Market Share: Growing market with steady progress
-
Key Drivers:
- Increasing cancer cases, improving healthcare infrastructure, rising awareness of nuclear medicine, and growing adoption of PET imaging technologies in urban centers.
- Trends: Increasing healthcare modernization
5. Middle East and Africa
- Market Share: Although small, but growing quickly
-
Key Drivers:
- Expanding diagnostic imaging capabilities, rising government investments in healthcare, growing prevalence of chronic diseases, and increased access to radiopharmaceuticals.
- Trends: Growing government initiatives for cancer diagnosis
Fluorine-18 Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is strong due to the presence of established players, such as Lantheus Holdings, Advanced Accelerator Applications, Curium Pharma, and Jubilant Pharma Limited, which are also adding to the competitive landscape across different regions.
This high level of competition urges companies to stand out by offering:
- Advanced security features
- Value-added services such as Analytics & predictive maintenance, real‑time operational analytics, and installation
- Competitive pricing models
- Strong customer support and easy integration
Opportunities and Strategic Moves
- Development of new F-18 labeled radiopharmaceuticals for diagnosis and targeted therapy in oncology, neurology, and cardiology is accelerating market growth. The expansion of PET imaging capabilities and the adoption of precision medicine are spurring demand for F-18 products, particularly as they enable early cancer detection and improved treatment monitoring.
- Leading industry players are establishing exclusive partnerships for technology transfer and regulatory support, notably in regions with high disease prevalence, such as China.
- Recent FDA and European approvals for novel F-18 tracers (e.g., flotufolastat, Pylclari) support enhanced imaging of prostate and cardiovascular conditions. Companies are investing in research to improve cyclotron yields, lower costs, and decentralize isotope production, aiding wider market access
Other companies analysed during the course of research:
- GE Healthcare
- Siemens Healthineers
- Cardinal Health
- Lantheus Medical Imaging
- Curium Pharma
- IBA Radiopharma Solutions
- Eckert & Ziegler Radiopharma
- Advanced Accelerator Applications
- Bracco Imaging
- Jubilant Radiopharma
- Positron Corporation
- Nordion (Canada) Inc.
- Blue Earth Diagnostics
- PETNET Solutions Inc.
- SOFIE Biosciences
Fluorine-18 Market News and Recent Developments
- Lantheus and GE HealthCare Announced Exclusive Licensing Agreement for Prostate Cancer Imaging Agent PYLARIFY in Japan Lantheus Holdings, Inc. and GE HealthCare announced an exclusive licensing agreement for GE HealthCare to develop, manufacture, and commercialize Lantheus’ piflufolastat F18 (PYLARIFY in the US market) in Japan for prostate cancer diagnostics and companion diagnostic use.
- GE HealthCare received FDA approval of Flyrcado (flurpiridaz F 18) injection PET radiotracer for enhanced diagnosis of coronary artery disease GE HealthCare’s FDA-approved flurpiridaz F 18 PET radiotracer, Flyrcado, delivers higher diagnostic efficacy in patients with known or suspected coronary artery disease (CAD), compared to SPECT MPI, the predominant procedure used in nuclear cardiology.
- Lantheus Receives U.S. FDA Approval of PYLARIFY (piflufolastat F 18) Injection, the First and Only Commercially Available PSMA PET Imaging Agent for Prostate Cancer Lantheus Holdings received the US Food and Drug Administration (FDA) approval for PYLARIFY, an F 18-labeled prostate-specific membrane antigen (PSMA) targeted positron emission tomography (PET) imaging agent to identify suspected metastasis or recurrence of prostate cancer. PYLARIFY is the first and only commercially available approved PSMA PET imaging agent for prostate cancer.
Fluorine-18 Market Report Coverage and Deliverables
The "Fluorine-18 Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Fluorine-18 Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Fluorine-18 Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Fluorine-18 Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the Fluorine-18 Market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For