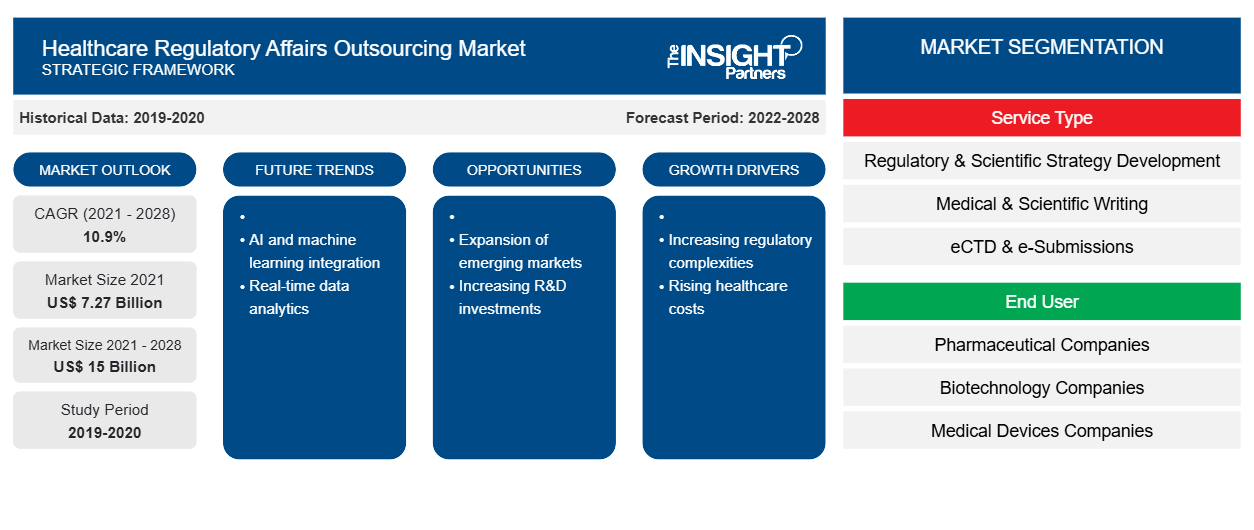
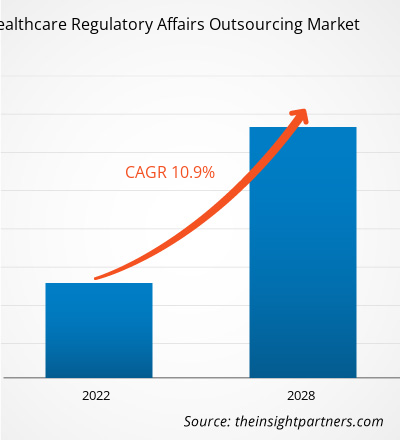
[Research Report] The healthcare regulatory affairs outsourcing market is projected to reach US$ 14,996.35 million by 2028 from US$ 7,274.73 million in 2021; it is expected to grow at a CAGR of 10.9 % from 2021 to 2028.
The increasing regulatory pressure on healthcare companies and escalating demand for speedy approval of new products. However, dearth of skilled professionals is restraining the healthcare regulatory affairs outsourcing market growth. Regulatory affairs outsourcing is the services offered to the pharmaceutical, biotech, and medical devices manufacturing industries. Regulatory affair outsourcing services help to achieve fast regulatory approvals. Regulatory affairs outsourcing industries are helping to get approval for new products, preparing protocols for conducting a clinical trial, publishing reports etc. An increase in demand for various services like regulatory consultation, medical writing and publishing of the regulatory documentation, clinical trial applications, and regulatory consulting and legal representations, patent application, product registration, and clinical trial applications has resulted in a surge in the adoption of healthcare regulatory affairs outsourcing business.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Healthcare Regulatory Affairs Outsourcing Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Healthcare Regulatory Affairs Outsourcing Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Increasing Regulatory Pressure on Healthcare Companies Healthcare Regulatory Affairs Outsourcing Market Growth
Continuous upgrades and progress in traditional drug development approaches are creating significant challenges in the healthcare sector. There is tremendous pressure on the pharmaceutical companies and medical fraternity to reduce the cost of prescription drugs, while their operational costs are skyrocketing. The complexity of regulatory requirements, declining revenues due to blockbuster drugs going off patent, and pressure from governments as well as health insurers for reduction in healthcare cost has presented additional challenges to healthcare industries. Given these difficulties, pharmaceutical companies have realized the need to leverage their resources along with the expertise provided by specialist external sources. Many high-end regulatory consulting companies are offering their expertise across the complete product life cycle. The outsourcing of regulatory affairs may enable sponsors to gain experience, optimize cost, and enhance productivity. Regulatory outsourcing companies are in better position to assess regulatory requirements, which allows them to select the best solutions. They are well versed with understanding associated with implementing, operating, and maintaining a regulatory publishing system. Most of the big pharmaceutical and biotechnology companies look out for consulting companies that can also offer supporting regulatory and pharmacovigilance services.
The increased complexity of regulatory filings underlines the demand for specialist CRO expertise. Having planned product-specific regulatory advice and strategies, along with healthcare regulatory compliance measures, in an early stages of product development is extremely important for the regulatory approval of the products. Failure to address the compliance in the early stage of development often leads to delay in the approval process due to inappropriately filed documentations, manufacturing oversights, omitted regulatory studies, and other failures to meet the regulatory requirements. Healthcare companies are now focusing on their core competencies and outsourcing the noncore functions to improve productivity and operational efficiency. They generally outsource regulatory functions to CROs operational in emerging markets, such as Asia Pacific and the MEA, which also allows them to reduce their operational costs and strengthen their focus on core functions such as R&D activities, and existing products’ sales and distribution.
Service Type-Based Insights
Based on service type, the healthcare regulatory affairs outsourcing market is segmented into Regulatory & Scientific Strategy Development, Medical & Scientific Writing, eCTD & e-Submissions, Data Management Services, Life Cycle Management Services, Pharmacovigilance, Chemistry Manufacturing & Controls (CMC) Services, Regulatory Labelling, Regulatory Artwork Services. The Medical & Scientific Writing segment is expected to hold a larger market share in 2021, and Pharmacovigilance segment is further anticipated to register a higher CAGR during the forecast period.
End User-Based Insights
Based on end user, the healthcare regulatory affairs outsourcing market is segmented into Pharmaceutical Companies, Biotechnology Companies, and Medical Devices Companies. The Pharmaceutical Companies segment would account for a larger market share in 2021. The market for the Pharmaceutical Companies segment is estimated to grow at a higher CAGR from 2021 to 2028.
Companies operating in the healthcare regulatory affairs outsourcing market adopt the product innovations strategy to meet the evolving customer demands worldwide, which also permits them to maintain their brand name in the global market.
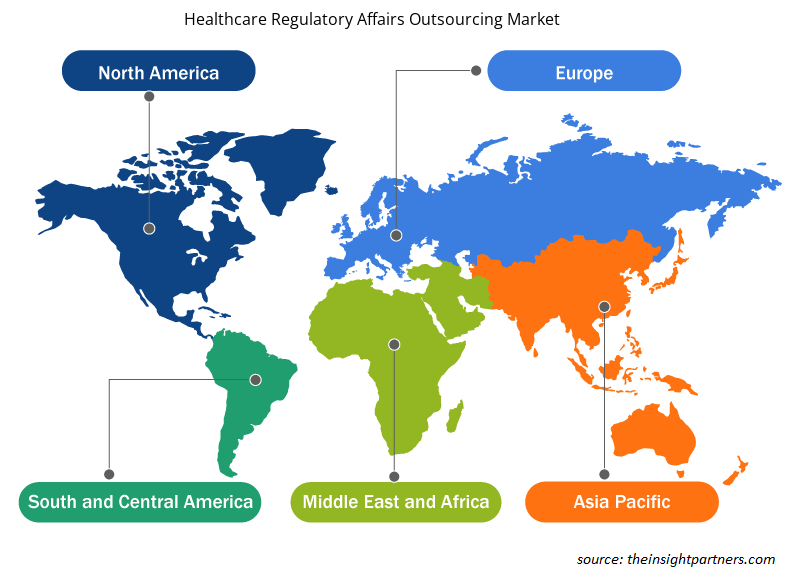
Healthcare Regulatory Affairs Outsourcing Market Regional Insights
The regional trends and factors influencing the Healthcare Regulatory Affairs Outsourcing Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Healthcare Regulatory Affairs Outsourcing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Healthcare Regulatory Affairs Outsourcing Market
Healthcare Regulatory Affairs Outsourcing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 7.27 Billion |
| Market Size by 2028 | US$ 15 Billion |
| Global CAGR (2021 - 2028) | 10.9% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Healthcare Regulatory Affairs Outsourcing Market Players Density: Understanding Its Impact on Business Dynamics
The Healthcare Regulatory Affairs Outsourcing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Healthcare Regulatory Affairs Outsourcing Market are:
- KLIFO
- ProPharma Group
- Arriello Ireland Ltd.
- DRA CONSULTING OY
- Asphalion S.L.
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Healthcare Regulatory Affairs Outsourcing Market top key players overview
Healthcare Regulatory Affairs Outsourcing Market – by Service Type
- Regulatory & Scientific Strategy Development
- Medical & Scientific Writing
- eCTD & e-Submissions
- Data Management Services
- Life Cycle Management Services
- Pharmacovigilance
- Chemistry Manufacturing & Controls (CMC) Services
- Regulatory Labelling
- Regulatory Artwork Services
Healthcare Regulatory Affairs Outsourcing Market – by End User
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Devices Companies
- Medical Device Software (SaMD)
- Medical Device Materials & Biomaterials
- Medical Device Biomarkers and In vitro Diagnostics (IVD)
- Medical Device Substance-based
- Medical Device of Combination Product (DDC)
Healthcare Regulatory Affairs Outsourcing Market – by Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- Spain
- Rest of Europe
- Asia Pacific (APAC)
- China
- India
- South Korea
- Japan
- Australia
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East & Africa
- South & Central America (SCAM)
- Brazil
- Argentina
- Rest of South and Central America
Company Profiles
- KLIFO
- ProPharma Group
- Arriello Ireland Ltd.
- DRA CONSULTING OY
- Asphalion S.L
- Parexel International Corporation
- IQVIA Inc.
- Pharmalex Gmbh
- ProductLife Group
- Voisin Consulting Life Sciences (VCLS)
- Azierta Contract Science Support Consulting
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Wind Turbine Composites Market
- Adaptive Traffic Control System Market
- Broth Market
- Biopharmaceutical Contract Manufacturing Market
- Transdermal Drug Delivery System Market
- Nuclear Decommissioning Services Market
- Electronic Health Record Market
- Fixed-Base Operator Market
- Power Bank Market
- Embolization Devices Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Service Type, End User and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, RoAPAC, RoSCAM, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
What is the regional market scenario of healthcare regulatory affairs outsourcing?
Global healthcare regulatory affairs outsourcing market is segmented by region into North America, Europe, Asia Pacific, Middle East & Africa and South & Central America. In North America, the U.S. is the largest market for anatomic pathology. The US is estimated to hold the largest share in the healthcare regulatory affairs outsourcing market during the forecast period. The growth of the market can be because of rise in cases of chronic diseases such as cancer, and increase in healthcare expenditure in the country. In addition, presence of major market players coupled with large number of funding programs by the public agencies in the region stimulate the growth of healthcare regulatory affairs outsourcing market in North America. On the other hand, enormous number of ongoing developments and innovations in healthcare, and rising spending capabilities in the Asia Pacific is expected to account for the fastest growth of the region during the coming years.
Who are the key players in the healthcare regulatory affairs outsourcing market?
The healthcare regulatory affairs outsourcing market majorly consists of the players such as Parexel International Corporation, IQVIA Inc., KLIFO, ProPharma Group, Arriello Ireland Ltd., DRA CONSULTING OY, Asphalion S.L, Pharmalex Gmbh, ProductLife Group, Voisin Consulting Life Sciences (VCLS), Azierta Contract Science Support Consulting among others.
Which end user held the largest share in the healthcare regulatory affairs outsourcing market?
The pharmaceutical companies segment dominated the global healthcare regulatory affairs outsourcing market and accounted for the largest revenue share of 43.0% in 2021.
What are the restraining factors for the healthcare regulatory affairs outsourcing market across the globe?
Key factors that are restraining the growth of this market are dearth of skilled professionals and data breach and lack of calibration.
What are healthcare regulatory affairs outsourcing?
Healthcare regulatory affairs mainly deal with the safety and efficacy of the product and other pharmaceutical agents. Medical writing and publishing of regulatory documentation prepared by experienced medical writers, quality control (QC) auditors, and publishers to develop high-quality documents for research projects regulatory submissions are among the services offered by healthcare regulatory affairs outsourcing. In addition, other regulatory outsourcing services include regulatory consulting, clinical trial applications, legal formalities, and guidelines, along with quality assurance and compliance.
What are the driving factors for the healthcare regulatory affairs outsourcing market across the globe?
Key factors that are driving the growth of this market are increasing regulatory pressure on healthcare companies and escalating demand for speedy approval of new products.
Which service type segment led the healthcare regulatory affairs outsourcing market?
The medical & scientific writing segment dominated the global anatomic pathology market and held the largest revenue share of 29.0% in 2021.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Healthcare Regulatory Affairs Outsourcing Market
- KLIFO
- ProPharma Group
- Arriello Ireland Ltd.
- DRA CONSULTING OY
- Asphalion S.L.
- Parexel International Corporation
- IQVIA Inc.
- Pharmalex Gmbh
- ProductLife Group
- Voisin Consulting Life Sciences (VCLS)
- Azierta Contract Science Support Consulting
 Get Free Sample For
Get Free Sample For