Embolization Devices Market Growth, Size, Share, Trends, Key Players Analysis, and Forecast till 2031
Embolization Devices Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (Embolization Coils, Plugs, Beads, Glues, and Others), Application (Neurology, Peripheral Vascular Disease, Oncology, Urology, and Others), End User (Hospitals, Ambulatory Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Jun 2025
- Report Code : TIPRE00008962
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 269
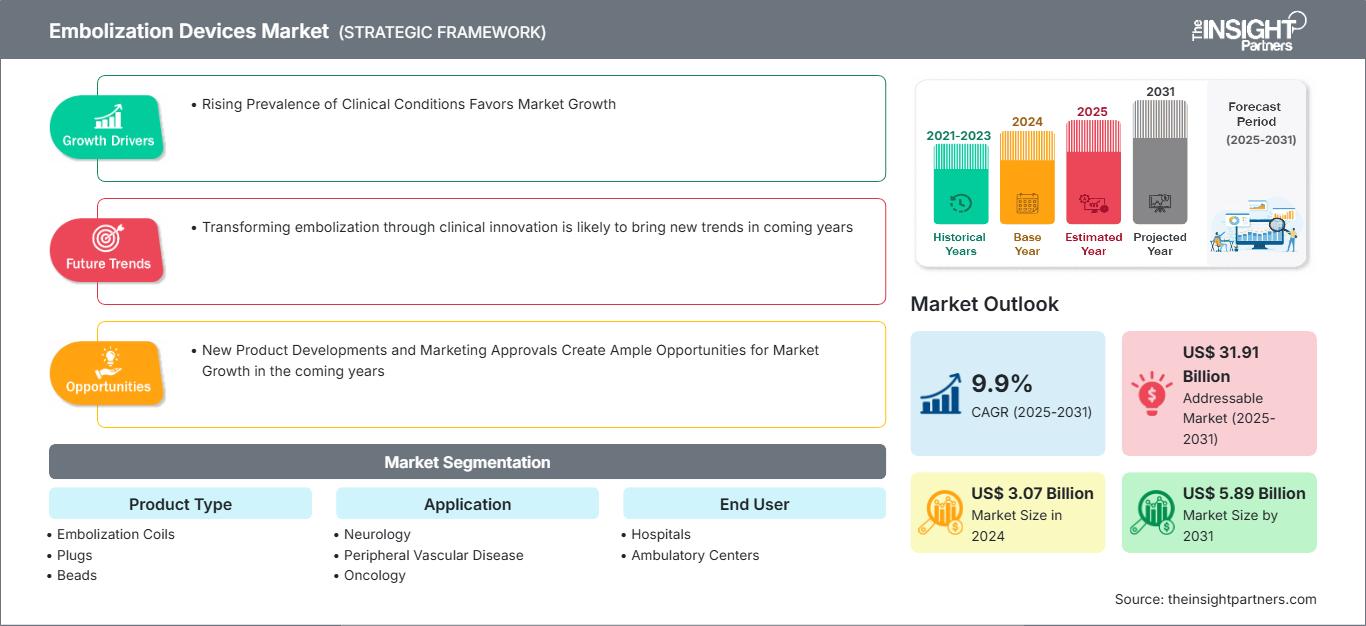
The embolization devices market size is projected to reach US$ 5.89 billion by 2031 from US$ 3.07 billion in 2024. The market is expected to register a CAGR of 9.9% during 2025-2031. Transforming embolization through clinical innovation is likely to bring new trends in the embolization devices market in the coming years.
Embolization Devices Market Analysis
The factors driving the embolization devices market include the rising prevalence of clinical conditions and burgeoning demand for minimally invasive techniques. Additionally, increasing awareness about various conditions and the development of new technologies are expected to contribute to market growth in the near future. Moreover, new product developments and marketing approvals are expected to create ample opportunities in the coming years.
Embolization Devices Market Overview
North America is projected to dominate the embolization devices market with the largest share during the forecast period. Further, Asia Pacific is expected to register a significant CAGR owing to the increasing prevalence of cancer and other clinical conditions, leading to a higher demand for interventional procedures. Technological advancements also played a crucial role in the market growth. Innovations in embolization devices, such as drug-eluting beads, microcatheters, and novel coil designs, have enhanced the efficacy and safety profiles of embolization procedures. These advancements not only improve clinical outcomes but also reduce procedure times and post-operative complications, making them more attractive among healthcare providers and patients. The shift toward minimally invasive surgical techniques is another significant factor bolstering the popularity of the embolization technique. Minimally invasive procedures aid in reduced hospital stays, quicker recovery times, and lower risk of complications compared to open surgeries. As healthcare providers and patients increasingly recognize these advantages, the demand for embolization procedures is expected to rise.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONEmbolization Devices Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Embolization Devices Market Drivers and Opportunities
Rising Prevalence of Clinical Conditions Favors Market Growth
Vascular diseases, including peripheral artery disease (PAD), aneurysms, and arteriovenous malformations (AVMs), are becoming more prevalent globally. These conditions have become particularly common among geriatric populations and individuals exposed to lifestyle-related risk factors. Peripheral vascular disease (PVD) consists of PAD and venous disease. According to the article "Income-Related Peripheral Artery Disease Treatment," published in the National Library of Medicine in 2022, more than 200 million people worldwide suffer from PAD, and ~10% of the affected people in Western Europe and North America were aged 50 years and above. The prevalence of PVD ranges from 3% in patients aged >55 years and 11% in patients aged >65 years to 20% in patients aged >75.
The cases of neurovascular conditions such as strokes and brain aneurysms are also on the rise across the world. As per the Brain Aneurysm Foundation estimates, 6.8 million people in the US have an unruptured brain aneurysm. Each year, nearly 30,000 Americans suffer a brain aneurysm rupture, often requiring immediate intervention through embolization techniques. Almost 500,000 people die worldwide due to brain aneurysms each year. Brain aneurysms are most prevalent in people from the age group of 35–60, and most aneurysms develop after the age of 40. Additionally, ruptured brain aneurysms account for 3–5% of all new strokes.
As per the World Health Organization (WHO), postpartum hemorrhage is the foremost cause of maternal mortality globally. Nearly 14 million women suffer from this condition each year, resulting in approximately 70,000 post-partem deaths worldwide. Additionally, as per the Institute for Functional Medicine, the prevalence of uterine fibroids has increased by 78.82% in the past 30 decades, growing from 126.41 million to 226.05 million in terms of number of cases. These clinical conditions can be effectively treated using embolization devices, which are designed to block abnormal or excessive blood flow within blood vessels. The technique is preferred due to its minimally invasive nature. Embolization involves the deliberate occlusion of blood vessels to treat conditions such as aneurysms, postpartum hemorrhage, uterine fibroids, and certain types of tumors. It is used to control severe bleeding after childbirth when conventional methods are ineffective, as well as block the blood supply to fibroids, causing them to shrink and symptoms to improve.
New Product Developments and Marketing Approvals Create Ample Opportunities for Market Growth
Small and big companies operating in the embolization devices market are increasingly adopting strategies such as geographic expansions, new product developments, and technological advancements to boost their revenues. A few of the noteworthy product developments and approvals in the embolization devices market, which are expected to drive its growth in the coming years, are mentioned below.
- In January 2024, the National Medical Products Administration provided marketing approval for the ZYLOX Phoenix Peripheral Detachable Fibered Coil Embolization System manufactured by Zylox-Tonbridge Medical Technology Co Ltd. This innovative, proprietary medical device has been designed for the minimally invasive interventional treatment of peripheral arterial embolism. Peripheral vascular intervention is the most prevalent application of this embolization coil system. Embolization treatment of visceral aneurysms, and the endoleak management of abdominal aortic aneurysms, hemoptysis, and arteriovenous fistula are the other important clinical applications of the system. With the regulatory approval for the use in these applications, the system has received international recognition for its safety and efficacy.
- In October 2023, Sirtex Medical launched the Lava Liquid Embolic System (LES), approved for the treatment of peripheral vascular hemorrhage. According to the company, Lava offers customizable volume and viscosity options to enable precise and controlled occlusion of target vessels. Available in 2-mL and 6-mL configurations, the system is specifically optimized for use in the peripheral vasculature. Its range of viscosities allows for effective distal embolization, reaching small and otherwise inaccessible vessels where other embolic agents may be less effective.
- In April 2022, Boston Scientific Corporation received 510(k) clearance from the US Food and Drug Administration (FDA) for the EMBOLD Fibered Detachable Coil designed to block or reduce the blood flow rate in the peripheral vasculature. As the newest addition to Boston Scientific's interventional oncology portfolio, the coil was designed for use in various embolization procedures, necessarily minimally invasive treatments intended to block one or more blood vessels to obstruct or reduce blood flow. The EMBOLD Fibered Detachable Coil features a single platform consisting of three coils, making it simpler for physicians to address various patient needs and anatomies.
- In March 2022, Artio Medical, Inc. received US FDA clearance for its Solus Gold Embolization Device, a next-generation solution for peripheral vascular occlusion. Designed to reduce or block blood flow within the peripheral vasculature, the device comes with a delivery system that combines flexibility with pushability, allowing physicians to navigate complex anatomies with ease.
Therefore, new product developments and marketing approvals are anticipated to create lucrative growth opportunities in the embolization devices market.
Embolization Devices Market Report Segmentation Analysis
Key segments that form the foundation of the embolization devices market analysis are product type, application, and end user.
- Based on product type, the embolization devices market is segmented into embolization coils, plugs, beads, glues, and others. The embolization coils segment held the largest market share in 2024 and is expected to register the highest CAGR during 2024–2031.
- By application, the embolization devices market is segmented into neurology, peripheral vascular disease, oncology, urology, and others. The neurology segment held the largest share of the market in 2024, and it is expected to register the highest CAGR in the market during 2024–2031.
- In terms of end user, the embolization devices market is segmented into hospitals, ambulatory centers, and others. The hospitals segment dominated the market in 2024 and is anticipated to register the highest CAGR during 2024–2031.
Embolization Devices Market Share Analysis by Geography
The geographical scope of the embolization devices market report is mainly divided into five regions: North America, Asia Pacific, Europe, and Middle East & Africa, and South & Central America. North America held a significant share of the market in 2024. The embolization devices market in North America is driven by the region’s advanced healthcare infrastructure, increasing prevalence of vascular diseases, and growing adoption of minimally invasive procedures. Technological advancements have led to a significant enhancement in the precision and safety of embolization techniques, making them a preferred choice for treating conditions such as aneurysms, arteriovenous malformations, and tumors. The high patient awareness about various medical conditions and treatment options, strong healthcare reimbursement systems, and the presence of leading medical device companies contribute to the market’s maturity in North America. Additionally, public health campaigns play a crucial role in promoting embolization procedures. Ongoing research and clinical trials are further expanding the scope of embolization technologies, with a particular focus on biodegradable and drug-eluting embolic agents. While the US leads in terms of adoption and innovation, Canada and Mexico are progressively increasing their market participation through supportive policies and infrastructure development. However, disparities in healthcare access across rural and urban settings remain a challenge. As a result, North American countries continue to attract significant investments, along with prompting collaborations among hospitals, research institutes, and device manufacturers, ensuring sustained development and innovation in embolization technologies.
Embolization Devices Market Regional InsightsThe regional trends and factors influencing the Embolization Devices Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Embolization Devices Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Embolization Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 3.07 Billion |
| Market Size by 2031 | US$ 5.89 Billion |
| Global CAGR (2025 - 2031) | 9.9% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Embolization Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Embolization Devices Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Embolization Devices Market top key players overview
Embolization Devices Market News and Recent Developments
The embolization devices market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A few of the developments in the market are listed below:
- Stryker has entered into a definitive agreement to acquire all of the issued and outstanding shares of common stock of Inari Medical, Inc. for US$ 80 per share in cash, representing a total fully diluted equity value of approximately US$ 4.9 billion. Inari will help Stryker gain a leading position in the fast-growing segment of venous thromboembolism (VTE). Inari’s innovative product portfolio complements Stryker’s Neurovascular business and includes mechanical thrombectomy solutions for peripheral vascular diseases such as deep vein thrombosis and pulmonary embolism. (Source: Stryker, Company Website, 2025)
- Boston Scientific Corporation has entered into a definitive agreement to acquire Intera Oncology Inc. Intera Oncology is a privately held medical device company that provides the Intera 3000 Hepatic Artery Infusion Pump and floxuridine (a chemotherapy drug), both of which are approved by the US Food and Drug Administration. The Intera 3000 pump is used to administer hepatic artery infusion (HAI) therapy to treat tumors in the liver primarily caused by metastatic colorectal cancer. (Source: Boston Scientific Corporation, Company Website, 2024)
- CERENOVUS Inc., a part of Johnson & Johnson MedTech, launched TRUFILL n-BCA Liquid Embolic System Procedural Set. With the latest addition, the CERENOVUS hemorrhagic stroke portfolio now offers TRUFILL n-BCA Liquid Embolic System as a new procedural set that includes two configurations and the accessories needed to undertake preparation and deliver n-BCA Liquid Embolic System in one sterilized set, creating streamlined procedure preparation. (Source: CERENOVUS Inc, Company Website, 2024)
Embolization Devices Market Report Coverage and Deliverables
The “Embolization Devices Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering below areas:
- Embolization devices market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Embolization devices market trends as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Embolization devices market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the embolization devices market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For