Healthcare Regulatory Affairs Outsourcing Market Segments and Growth by 2028
Healthcare Regulatory Affairs Outsourcing Market Forecast to 2028 - COVID-19 Impact and Global Analysis By Service Type (Regulatory & Scientific Strategy Development, Medical & Scientific Writing, eCTD & e-Submissions, Data Management Services, Life Cycle Management Services, Pharmacovigilance, Chemistry Manufacturing & Controls (CMC) Services, Regulatory Labelling, Regulatory Artwork Services); End User (Pharmaceutical Companies, Biotechnology Companies, Medical Devices Companies) and Geography
Historic Data: 2019-2020 | Base Year: 2021 | Forecast Period: 2022-2028- Report Date : Oct 2021
- Report Code : TIPRE00007611
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 192
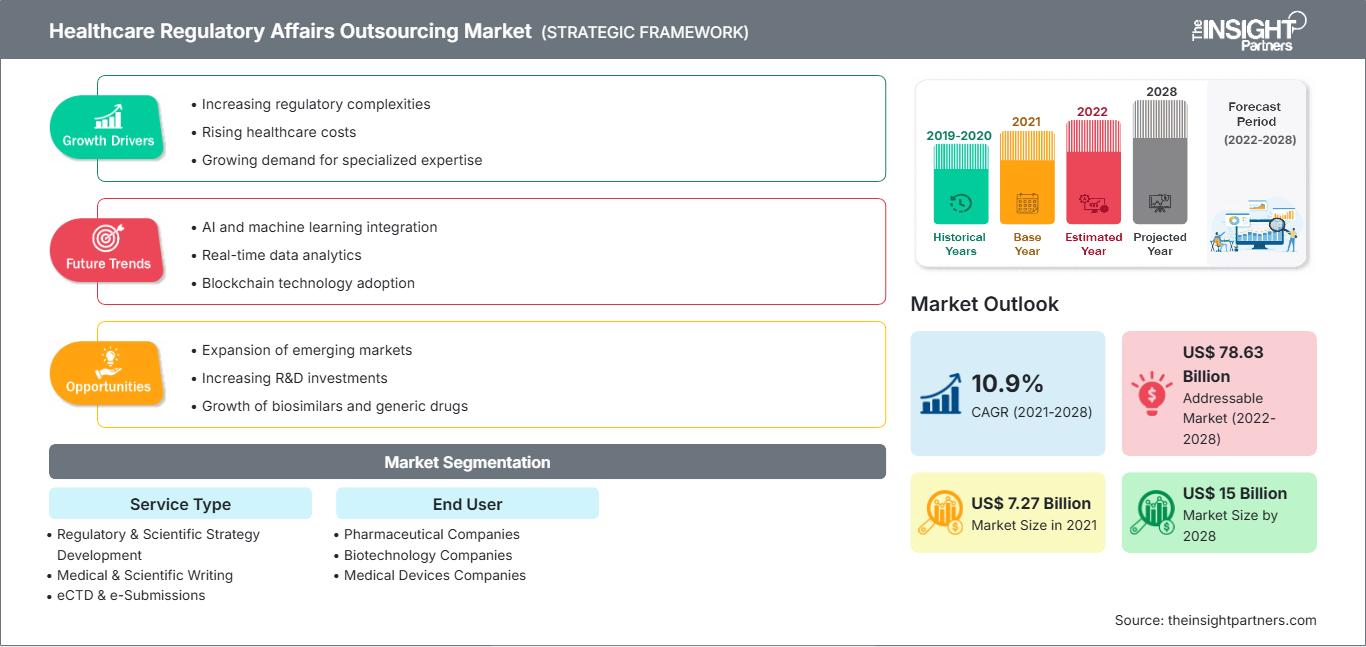
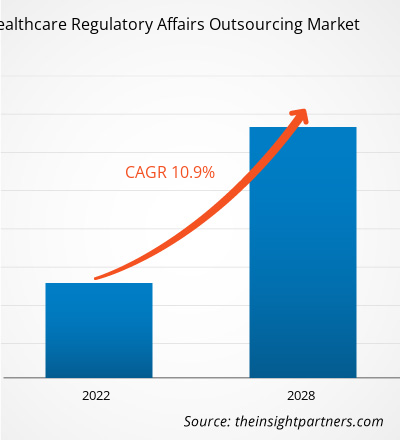
[Research Report] The healthcare regulatory affairs outsourcing market is projected to reach US$ 14,996.35 million by 2028 from US$ 7,274.73 million in 2021; it is expected to grow at a CAGR of 10.9 % from 2021 to 2028.
The increasing regulatory pressure on healthcare companies and escalating demand for speedy approval of new products. However, dearth of skilled professionals is restraining the healthcare regulatory affairs outsourcing market growth. Regulatory affairs outsourcing is the services offered to the pharmaceutical, biotech, and medical devices manufacturing industries. Regulatory affair outsourcing services help to achieve fast regulatory approvals. Regulatory affairs outsourcing industries are helping to get approval for new products, preparing protocols for conducting a clinical trial, publishing reports etc. An increase in demand for various services like regulatory consultation, medical writing and publishing of the regulatory documentation, clinical trial applications, and regulatory consulting and legal representations, patent application, product registration, and clinical trial applications has resulted in a surge in the adoption of healthcare regulatory affairs outsourcing business.
Customizee This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONHealthcare Regulatory Affairs Outsourcing Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Increasing Regulatory Pressure on Healthcare Companies Healthcare Regulatory Affairs Outsourcing Market Growth
Continuous upgrades and progress in traditional drug development approaches are creating significant challenges in the healthcare sector. There is tremendous pressure on the pharmaceutical companies and medical fraternity to reduce the cost of prescription drugs, while their operational costs are skyrocketing. The complexity of regulatory requirements, declining revenues due to blockbuster drugs going off patent, and pressure from governments as well as health insurers for reduction in healthcare cost has presented additional challenges to healthcare industries. Given these difficulties, pharmaceutical companies have realized the need to leverage their resources along with the expertise provided by specialist external sources. Many high-end regulatory consulting companies are offering their expertise across the complete product life cycle. The outsourcing of regulatory affairs may enable sponsors to gain experience, optimize cost, and enhance productivity. Regulatory outsourcing companies are in better position to assess regulatory requirements, which allows them to select the best solutions. They are well versed with understanding associated with implementing, operating, and maintaining a regulatory publishing system. Most of the big pharmaceutical and biotechnology companies look out for consulting companies that can also offer supporting regulatory and pharmacovigilance services.
The increased complexity of regulatory filings underlines the demand for specialist CRO expertise. Having planned product-specific regulatory advice and strategies, along with healthcare regulatory compliance measures, in an early stages of product development is extremely important for the regulatory approval of the products. Failure to address the compliance in the early stage of development often leads to delay in the approval process due to inappropriately filed documentations, manufacturing oversights, omitted regulatory studies, and other failures to meet the regulatory requirements. Healthcare companies are now focusing on their core competencies and outsourcing the noncore functions to improve productivity and operational efficiency. They generally outsource regulatory functions to CROs operational in emerging markets, such as Asia Pacific and the MEA, which also allows them to reduce their operational costs and strengthen their focus on core functions such as R&D activities, and existing products’ sales and distribution.
Service Type-Based Insights
Based on service type, the healthcare regulatory affairs outsourcing market is segmented into Regulatory & Scientific Strategy Development, Medical & Scientific Writing, eCTD & e-Submissions, Data Management Services, Life Cycle Management Services, Pharmacovigilance, Chemistry Manufacturing & Controls (CMC) Services, Regulatory Labelling, Regulatory Artwork Services. The Medical & Scientific Writing segment is expected to hold a larger market share in 2021, and Pharmacovigilance segment is further anticipated to register a higher CAGR during the forecast period.
End User-Based Insights
Based on end user, the healthcare regulatory affairs outsourcing market is segmented into Pharmaceutical Companies, Biotechnology Companies, and Medical Devices Companies. The Pharmaceutical Companies segment would account for a larger market share in 2021. The market for the Pharmaceutical Companies segment is estimated to grow at a higher CAGR from 2021 to 2028.
Companies operating in the healthcare regulatory affairs outsourcing market adopt the product innovations strategy to meet the evolving customer demands worldwide, which also permits them to maintain their brand name in the global market.
Healthcare Regulatory Affairs Outsourcing Market Regional Insights
The regional trends and factors influencing the Healthcare Regulatory Affairs Outsourcing Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Healthcare Regulatory Affairs Outsourcing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Healthcare Regulatory Affairs Outsourcing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 7.27 Billion |
| Market Size by 2028 | US$ 15 Billion |
| Global CAGR (2021 - 2028) | 10.9% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Healthcare Regulatory Affairs Outsourcing Market Players Density: Understanding Its Impact on Business Dynamics
The Healthcare Regulatory Affairs Outsourcing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Healthcare Regulatory Affairs Outsourcing Market top key players overview
Healthcare Regulatory Affairs Outsourcing Market – by Service Type
- Regulatory & Scientific Strategy Development
- Medical & Scientific Writing
- eCTD & e-Submissions
- Data Management Services
- Life Cycle Management Services
- Pharmacovigilance
- Chemistry Manufacturing & Controls (CMC) Services
- Regulatory Labelling
- Regulatory Artwork Services
Healthcare Regulatory Affairs Outsourcing Market – by End User
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Devices Companies
- Medical Device Software (SaMD)
- Medical Device Materials & Biomaterials
- Medical Device Biomarkers and In vitro Diagnostics (IVD)
- Medical Device Substance-based
- Medical Device of Combination Product (DDC)
Healthcare Regulatory Affairs Outsourcing Market – by Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- Spain
- Rest of Europe
- Asia Pacific (APAC)
- China
- India
- South Korea
- Japan
- Australia
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East & Africa
- South & Central America (SCAM)
- Brazil
- Argentina
- Rest of South and Central America
Company Profiles
- KLIFO
- ProPharma Group
- Arriello Ireland Ltd.
- DRA CONSULTING OY
- Asphalion S.L
- Parexel International Corporation
- IQVIA Inc.
- Pharmalex Gmbh
- ProductLife Group
- Voisin Consulting Life Sciences (VCLS)
- Azierta Contract Science Support Consulting
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Related Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For