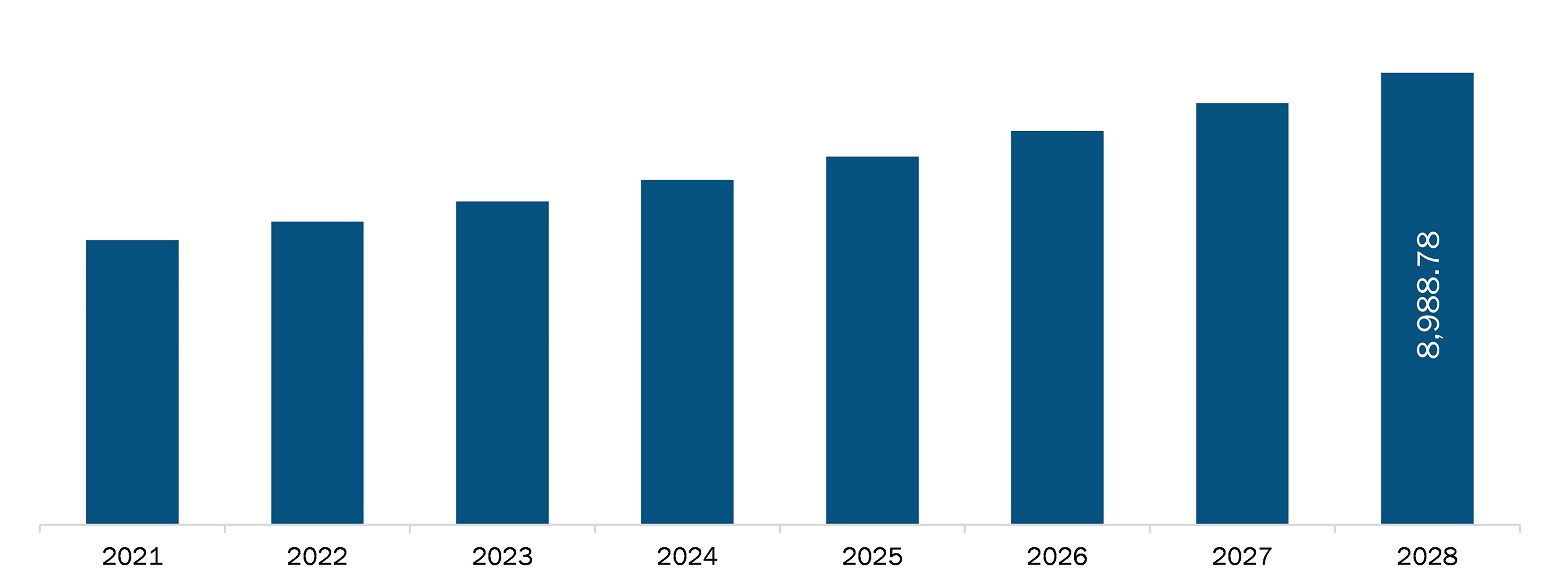
The North America laboratory developed test market is expected to reach US$ 8,988.78 million by 2028 from an estimated value of US$ 5,664.43 million in 2021; it is likely to register a CAGR of 6.8% from 2021 to 2028.
The key factors driving the growth of market are increasing incidence of cancer and genetic disorders and a large number of product launches for laboratory developed tests. However, the changing regulatory landscape may hamper the growth of the North America laboratory development test market during the forecast period.
Cancer is one of the leading causes of death worldwide. As per the WHO estimates, cancer was a cause of ~9.6 million deaths in 2018. Clinical diagnostics helps in detecting early signs and risk factors, paving way for early intervention. The laboratory developed tests (LDTs) have a decisive impact on each step of diagnosis, from screening to the prevention of certain diseases and early diagnosis at the onset of illness. For instance, the Oncotype DX lab test is used to determine if chemotherapy would benefit patients with early-stage breast cancer. It also helps evaluate the likelihood of disease recurrence; for this, the test is performed on a small tissue sample removed during breast cancer surgery. In addition, the increasing incidence of genetic disorders is also driving the demand for LDTs. A large number of LDTs are available for genetic testing owing to a lack of availability of genetic tests in the market. As per the Association for Molecular Pathology 2018 assessment, ~70,000 genetic tests are available in the medical market, and most of these are LDTs. Therefore, the rising incidence of cancer and growing awareness regarding the importance of early diagnostics are boosting the adoption of LDTs. Also, LDTs are developed and used within laboratories and are not distributed or sold to other laboratories or healthcare facilities. These tests are developed to overcome the challenge of the unavailability of commercial tests. Many LDTs are genetic tests developed for rare diseases. Thus, the frequency of development and introduction of new LDTs is high. Also, the increasing emergence of SARS-CoV-2 variants has highlighted the need to identify, trace, and track mutations across the complete viral genome. Hence, increasing research on the development of LDTs to detect cancer and autoimmune diseases is driving the market growth.
The COVID-19 pandemic has emphasized a need for widespread diagnostic testing. The commercially available test kits have proved insufficient to meet the demand owing to manufacturing, supply chain limitations, and the timeline required for Food and Drug Administration (FDA) review and authorization of test products. In contrast, laboratory developed tests (LDTs) are internally developed and validated at the performing laboratory allowed laboratories to fill the testing gaps during the pandemic and in other clinical scenarios where commercial tests are scarce. The government regulation efforts have also bolstered the laboratory developed tests growth since the pandemic. In August 2020, to address the COVID-19 testing shortage, the US Department of Health and Human Services (HHS) made a brief statement that effectively rescinded the FDA guidance requiring clinical labs to seek an emergency use authorization (EUA) or submit data to the FDA in conjunction with offering laboratory-developed tests (LDTs).
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
NORTH AMERICA LABORATORY DEVELOPED TEST MARKET SEGMENTATION
By Type
- Clinical Biochemistry
- Critical Care
- Haematology
-
- Coagulation and Hemostasis,
- Hemoglobin Testing
- Blood Count Testing
- Others
- Immunology
- Microbiology
- Molecular Diagnostics
- Other Test Types
By Application
- Academic Institutes
- Clinical Research organizations
- Hospitals laboratory
- Specialty Diagnostic Centers
- Others
By Country
- US
- Canada
- Mexico
Company Profiles
- Quest Diagnostics Incorporated
- F. HOFFMANN-LA ROCHE LTD
- QIAGEN
- Illumina, Inc.
- Eurofins Scientific
North America Laboratory Developed Test Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 5,664.43 Million |
| Market Size by 2028 | US$ 8,988.78 Million |
| CAGR (2021 - 2028) | 6.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For