North America, the pharmaceutical continuous manufacturing market, is anticipated to reach US$ 1,335.15 Mn in 2027 from US$ 619.24 Mn in 2019. The market is projected to grow with a CAGR of 10.2% from 2020-2027.
The pharmaceutical continuous manufacturing market is growing primarily due to the advantages offered by continuous manufacturing process. Restraining factor such as, high capital investments is likely to damage the growth of the market in the coming years. On the other hand, increasing R&D spending is expected to have a positive impact on the growth of the North America pharmaceutical continuous manufacturing market in the coming years.
Pharmaceutical and medical devices companies are focusing on R&D to develop new molecules for various applications and drug discovery platforms. The companies invest majorly on R&Ds with an aim to deliver high quality and innovative products to the market. R&D spending by the biopharmaceutical companies has also increased over the years. According to a report of Pharmaceutical Research and Manufacturers of America (PhRMA), the R&D expenditure of the biopharmaceutical companies has increased from US$ 49.6 billion 2012 to US$ 58.8 billion in 2015. Also, Evonik Corporation has invested more than US$ 50 million over the last 4 years to advance its competencies in medical devices. Additionally, the companies are engaged in establishing new collaborations and partnerships for the development of new drug manufacturing.
Thus, the growing R&D expenditure drives the pharmaceutical continuous manufacturing market during the forecast period.
The number of infections of COVID-19 has killed thousands and rising exponentially in the region. The US is one of the biggest markets for new product developments, along with the adoption of continuous pharmaceutical manufacturing in companies. Pharmaceutical supplies and procurement are also severely affected by the outbreak of COVID-19 in North America. The coronavirus disease 2019 (COVID-19) outbreak highlights potential vulnerabilities in both our drug supply chain and our clinical infrastructure, including the availability of adequate clinician resources.
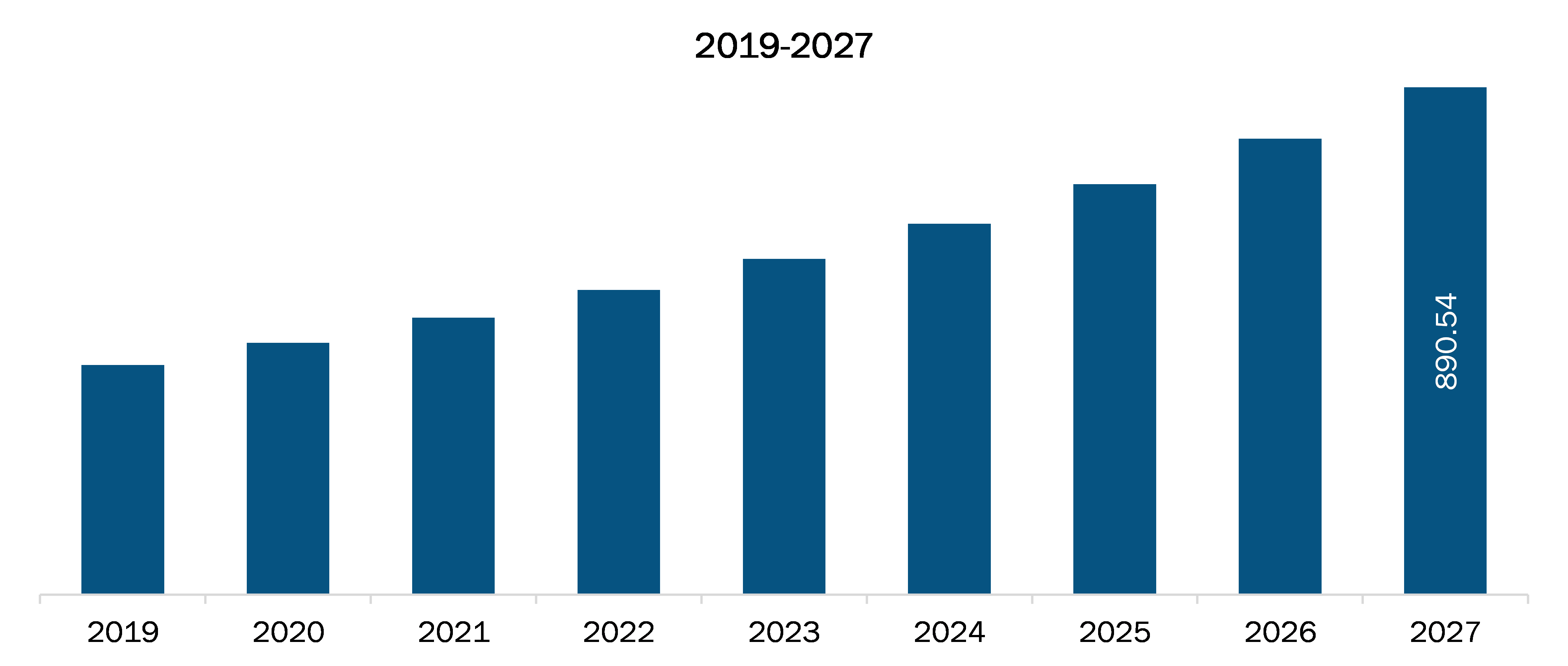
In 2019, the US accounted for the largest market share in North America pharmaceutical continuous manufacturing market, and it is also expected to grow at a faster pace over the forecast period. Drug approval received by FDA is the key factors that are responsible for the highest revenue share of the region. For instance, in April 2016, the production of Janssen’s Prezista that switched from batch to continuous manufacturing processing received approval from the US FDA. Moreover, the US FDA also undertook various initiatives to promote continuous manufacturing in the country within the pharmaceutical industrial space. Hence, considering the mentioned factors, the pharmaceutical continuous manufacturing market is expected to grow at a significant pace in the US during the forecast period.
US Pharmaceutical Continuous Manufacturing Market Revenue and Forecasts to 2027 (US$ Mn)
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
NORTH AMERICA PHARMACEUTICAL CONTINUOUS MANUFACTURING – MARKET SEGMENTATION
By Product
- Integrated Systems
- Semi-Continuous Systems
- Continuous Granulators
- Continuous Blenders
- Continuous Compressors
- Continuous Coaters
- Continuous Dryers
- Other Semi-Continuous Systems
- Controls
By Application
- End Product Manufacturing
- Solid Dosage Manufacturing
- Liquid Dosage Manufacturing
- API Manufacturing
By End-User
- Full Scale Manufacturing Companies
- Pharmaceutical Companies
- Contract Manufacturing Organizations
- R & D Departments
- Contract Research Organizations
- Research Institutes
By Geography
- North America
-
- US
- Canada
- Mexico
Company Profiles
- Munson Machinery Co., Inc.,
- THERMO FISHER SCIENTIFIC INC.
- GEA Group
- Coperion GmbH
- Gericke AG
North America Pharmaceutical Continuous Manufacturing Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 619.24 Million |
| Market Size by 2027 | US$ 1,335.15 Million |
| CAGR (2020 - 2027) | 10.2% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For