The North America pulmonary devices market is expected to reach US$ 16,637.44 million by 2027 from US$ 7,581.04 million in 2019; it is estimated to grow at a CAGR of 10.3% from 2020 to 2027.
Increasing prevalence of respiratory diseases and growing number of COVID-19 in the region are the key factors driving the growth of pulmonary devices. However unfavorable reimbursement scenario is the major factor hindering the market growth in North America.
Pulmonary or respiratory devices are used to remove mucus and secretions from the airways or the respiratory tract. These medical devices are focused on diagnosis, control, treatment, management, and evaluation of the problems associated with respiratory tract.
Manufacturers in the pulmonary devices market are ramping up their focus on the adoption of various strategies, such as product innovation, product launches, and approvals, as well as R&D investment for advancements in ventilators, along with merger and acquisition as their developmental strategies to maintain the competitive environment in the market.
For instance, in January 2020, Masimo Corporation acquired Connected Care Business from NantHealth (US). This acquisition will support Masimo to assist hospitals in providing constant care through connectivity, automation, and innovative noninvasive monitoring technologies. Similarly, in April 2019, Inogen, Inc. introduced the Inogen One G5 portable oxygen concentrator with the highest oxygen production capacity per pound of weight, amounting to 1,260 ml. In May 2020, Philips partnered with Masimo. This partnership was aimed at integrating Masimo’s measurement technologies into select intellivue MX-Series multi-parameter monitors to help clinicians examine ventilation status and cerebral oximetry. In March 2019, ResMed acquired HB Healthcare Safety, a provider of home-based medical equipment for sleep and respiratory care devices.
The impact of the COVID-19 crisis is expected to further accelerate the growth of the respiratory devices market in North America. In addition, the threat will have a lasting impact on governments and regulatory agencies in terms of future preparedness when it comes to the next pandemic. Today, government is playing catch-up, swiftly intervening in an attempt to create alliances with manufacturers to handle the COVID-19 threat. In addition, international regulatory agencies are considering accelerated approval of certain respiratory devices.
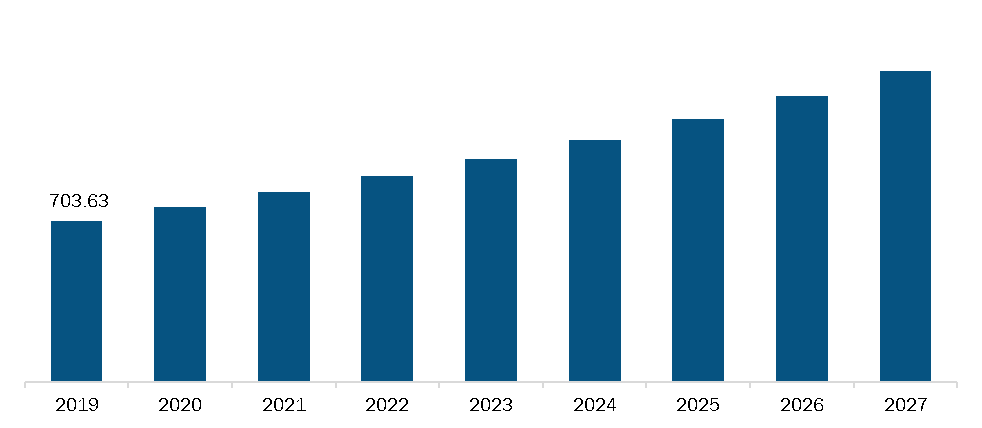
Mexico Pulmonary Devices Market, Revenue and Forecast to 2027 (US$ Mn)
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
NORTH AMERICA PULMONARY DEVICES MARKET SEGMENTATION
By Type
- Therapeutic Devices
- Consumables and Accessories
- Diagnostic Devices
- Monitoring Devices
By Application
- Chronic Obstructive Pulmonary Disease (COPD)
- Sleep Apnea
- Asthma
- Infectious Diseases
- Others
By End User
- Hospitals
- Home Care Settings
- Ambulatory Care Centers
By Country
- US
- Canada
- Mexico
Company Profiles
- ResMed
- Masimo
- Invacare Corporation
- Koninklijke Philips N.V.
- Getinge AB
North America Pulmonary Devices Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 7,581.04 Million |
| Market Size by 2027 | US$ 16,637.44 Million |
| CAGR (2020 - 2027) | 10.3% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For