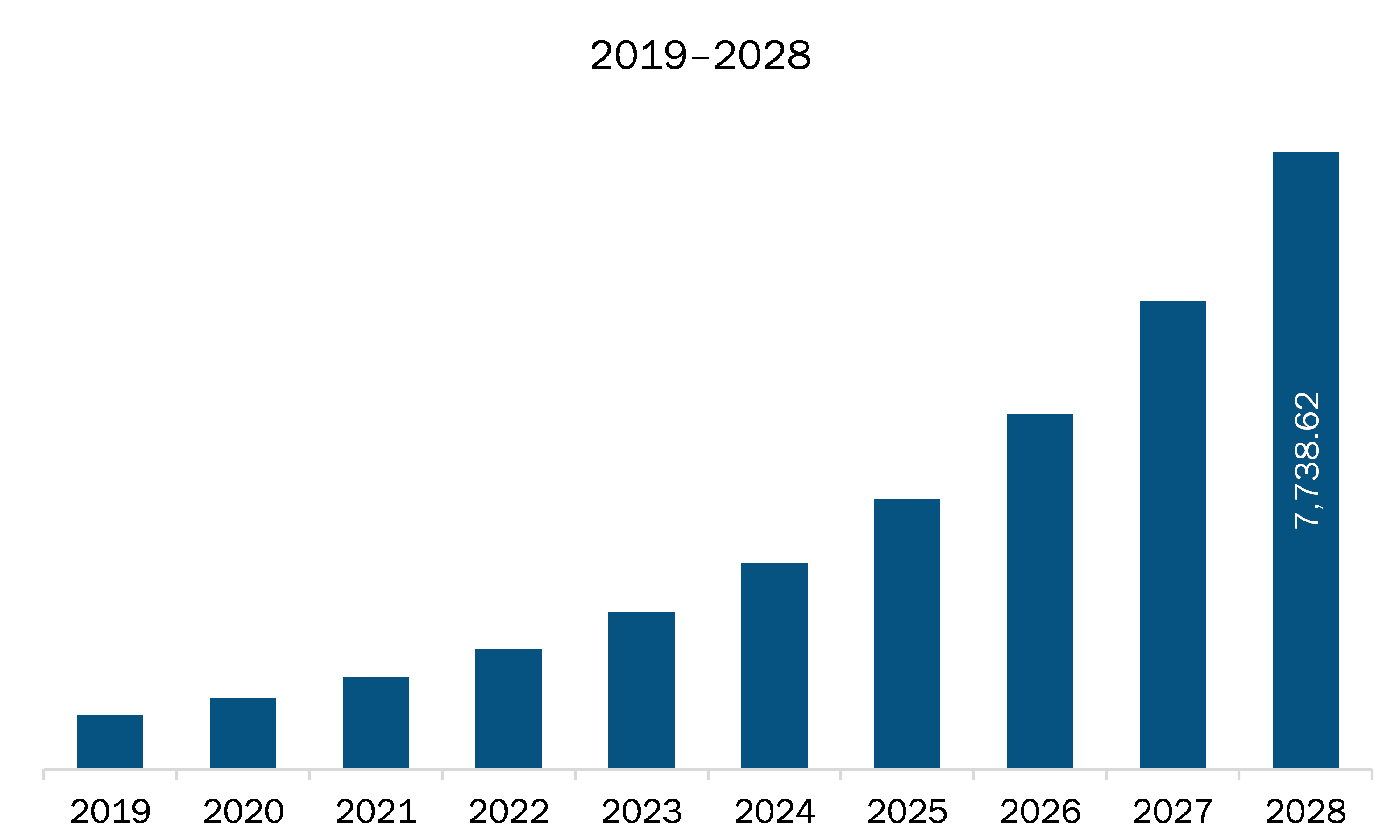
The remote cardiac monitoring market in North America is expected to grow from US$ 1,153.06 million in 2021 to US$ 7,738.62 million by 2028; it is estimated to grow at a CAGR of 31.3% from 2021 to 2028.
The US, Canada, and Mexico are major economies in North America. Continuous developments in telemedicine approach is the major factor driving the growth of the North America remote cardiac monitoring market. More than 85 million Americans, ~26% of the US population, suffer from CVDs, and ~7 million, i.e., 2.2% of the population, survive stroke. CVDs and stroke cost the US healthcare system more than US$ 320 billion and US$ 33 billion, respectively, each year. By 2030, the annual costs of CVDs and stroke are projected to balloon to nearly US$ 1 trillion. Thus, there is an urge to design strategies to increase the value of health care by enhancing the quality of care and lowering costs; offering patients better access to care, via telehealth, can help address this need. As defined by Office for the Advancement of Telehealth, telehealth comprises the use of telecommunications and information technologies to share information as well as to remotely provide clinical care, health education, public health, and administrative services. It has the potential to help transform healthcare systems, simultaneously reducing costs as well as boosting quality, patient-centeredness, and patient satisfaction. To successfully treat heart-related diseases, including CVDs, health professionals encourage patients to make lifestyle changes, continue a regular course of medication, and rigorously follow the follow-up schedule. However, despite the potential health benefits of regular doctor appointments and tests, clinic visits may act as an imposition on patients’ daily lives. As a result, disruptions in follow-up have become a common issue. Further, this situation has worsened with the pandemic. Clinic visits and elective procedures were suspended, and patients avoided visiting clinics due to the fear of SARS-CoV-2 infection. As per the American College of Cardiology, the pandemic has disrupted every aspect of cardiovascular care delivery, and the adverse effects on the same would be last long if health systems fail to adapt to the changes quickly and efficiently. Telehealth helps health systems in overcoming a few of the systemic barriers in monitoring heart diseases and CVDs. Technological solutions address the challenges posed by physical distance and facilitate personal interactions between doctors and patients. The doctors can remotely monitor conditions that contribute to heart attack and stroke, providing an opportunity to implement primary and secondary cardiovascular prevention without asking patients to visit the clinics. Patients can get any tests done before the appointment at a lab close to their home; they can also measure their blood pressure with an automatic blood pressure cuff at home, local pharmacies, or senior centers. Telehealth appointments are more convenient than in-person clinic visits for both doctors and patients. The introduction of remote monitoring of health data using wireless devices that measure weight, blood pressure, blood sugar, pulse, and heart rhythm could further advance telehealth services. Moreover, the telemedicine approach uses remote patient monitoring systems or devices to keep a check on the health status of patients. Along with cardiac monitors, companies are also working on developing monitoring devices for hematology, including blood glucose monitors, and for respiratory diseases, including sleep apnea and respiratory rate monitors, which is further anticipated to drive the Remote Cardiac Monitoring market in North America.
Since the COVID-19 pandemic, the US has suspended all the non-urgent scheduled visits and hospital stays. They have transitioned to virtual health platforms, using wearable sensors at unprecedented rates to reduce virus exposure and the use of limited stocks of personal protective equipment. The FDA has also issued emergency clearance for multiple RPM devices to enable further physiological data collection in this age of social distancing. The COVID-19 pandemic has ushered in a new era of heart management with remote cardiac monitoring. These remote cardiac device monitors changed the game's rules during the COVID-19 pandemic by redefining how patients use the devices to interact with their caregivers. By recording the device and physiological data timely and then sending that data securely over the internet to a patient's health team, countless patients have been able to leave the emergency room, avoid in-person clinic visits, and thus reduce exposure to the novel coronavirus. The ability to remotely monitor cardiac devices has given patients confidence in an already stressful time. It has helped nurses free up more hospital beds, at the same time reducing the need for expensive and sometimes scarce protective equipment.The response to COVID-19 across Canada has resulted in physical distancing protocols in hospitals, and equipment clinics have responded with increased remote monitoring. There is a need to assess how changes in the health landscape due to COVID-19 have affected cardiovascular implantable electronic device (CIED) follow-up care for patients and device clinics in Canada and investigate whether changes are temporary or permanent. The lack of a unified, process-specific, and guideline-based approach to post-implantation care of cardiovascular implantable electronic devices (CIEDs) and inconsistent funding guidelines in all jurisdictions for hospital and remote visits are significant obstacles to the use of remote patient management strategies in Canada.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
North America Remote Cardiac Monitoring Market Segmentation
North America Remote Cardiac Monitoring Market – By Product Type
- Devices
- Vital Signs Monitors
- Heart Rate Monitors
- Blood Pressure Monitors
- Breath Monitors
- Holter Monitors
- Others
- Software
- Cloud Based Software
- On-Premise Software
- Services
North America Remote Cardiac Monitoring Market – By End User
- Hospitals and Clinics
- Emergency Settings
- Homecare Settings
- Others
North America Remote Cardiac Monitoring Market– By Country
- US
- Canada
- Mexico
North America Remote Cardiac Monitoring Market-Companies Mentioned
- Abbott
- AMC Health
- Biotronik, Inc.
- Boston Scientific Corporation
- GE Healthcare
- Honeywell International Inc.
- Koninklijke Philips N.V.
- Medtronic
- Nihon Kohden Corporation
- OSI Systems, Inc.
North America Remote Cardiac Monitoring Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 1,153.06 Million |
| Market Size by 2028 | US$ 7,738.62 Million |
| CAGR (2021 - 2028) | 31.3% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For