Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Insights 2025-2031
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Techniques (Electroencephalography (EEG), Brain-computer interface (BCI), Eye tracking and near-infrared spectroscopy (NIRS), Magnetic resonance imaging (MRI), Magnetoencephalography (MEG), Transcranial magnetic stimulation (TMS), Cerebral Metabolic Rate of Oxygen (CMRO2), Intracranial Pressure, and Others), Non-invasive ICP (Transcranial Doppler Ultrasonography (TCD), Tympanic Membrane Displacement (TMD), Optic Nerve Sheath Diameter (ONSD), Magnetic Resonance Imaging (MRI) and Computer Tomography (CT), and Fundoscopy and Papilledema), Device (Imaging devices and Monitoring Devices), End User (Hospitals and Clinics, Diagnostic Centers and Others), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Oct 2025
- Report Code : TIPRE00038985
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 327
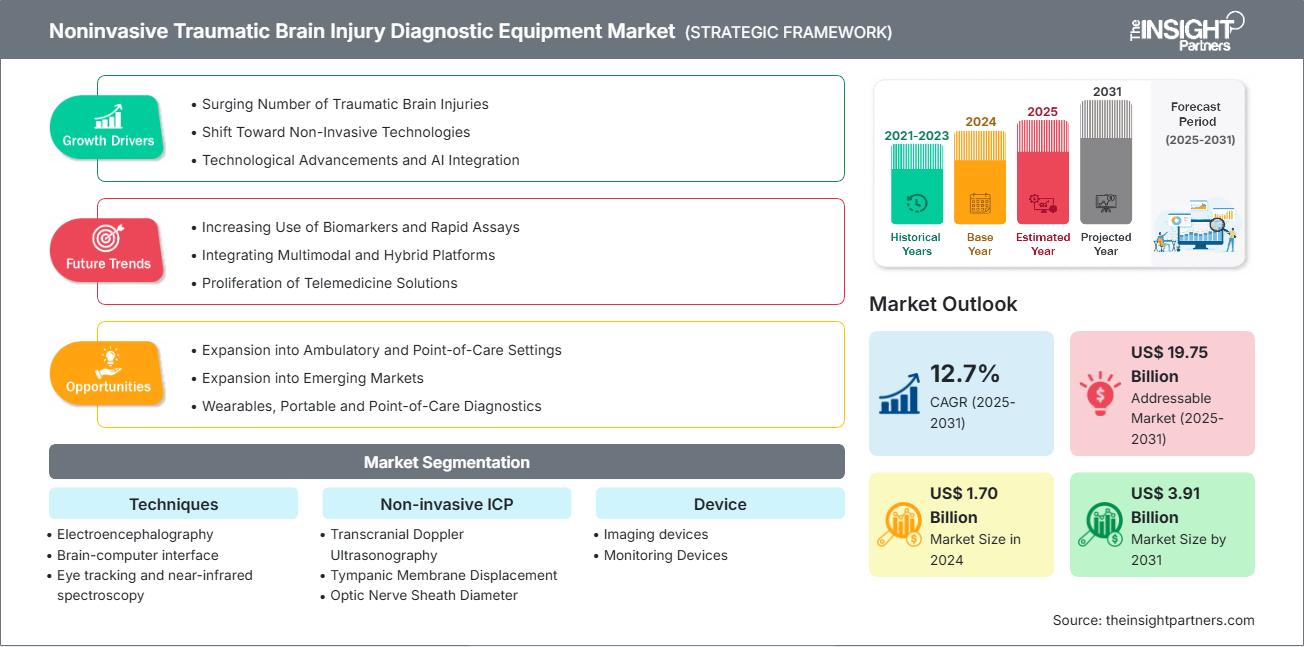
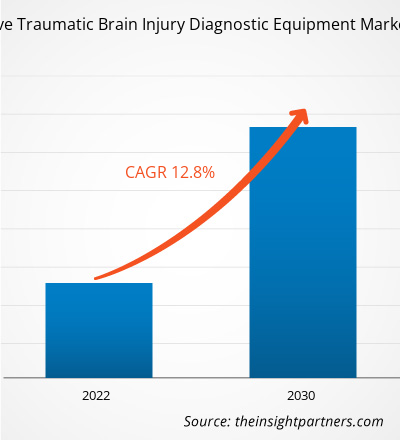
The noninvasive traumatic brain injury diagnostic equipment market is projected to reach US$ 3.91 billion by 2031, up from US$ 1.70 billion in 2024, and register a CAGR of 12.7% from 2025 to 2031.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Analysis
The surging number of traumatic brain injuries, a shift toward noninvasive technologies, technological advancements, and AI integration drive market growth. The expansion into ambulatory and point-of-care settings, as well as emerging markets, will create ample opportunities for the noninvasive traumatic brain injury diagnostic equipment market in the coming years.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Overview
North America is projected to dominate the market, accounting for the largest share during the forecast period. Asia Pacific is expected to register a significant CAGR during the forecast period, owing to the surging number of traumatic brain injuries. Additionally, advanced healthcare infrastructure, high prevalence of TBI, and commitment to research and development fuel demand for noninvasive traumatic brain injury diagnostic equipment.
Customizee This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONNoninvasive Traumatic Brain Injury Diagnostic Equipment Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Drivers and Opportunities
Market Drivers:
- Surging Number of Traumatic Brain Injuries: The surging disease burden gives rise to significant clinical, social, and economic needs. Mild traumatic brain injuries (TBIs) are estimated to account for approximately 50% of all TBIs globally.
- Shift Toward Noninvasive Technologies: Portable imaging technologies, including EEG, NIRS, MRI, and AI-powered ultrasound, are transforming diagnostics, setting a new benchmark for innovation and speed in the healthcare market.
- Technological Advancements and AI Integration: There is a growing demand for tools that analyze data, reduce human error, shorten diagnosis time, and push diagnostics closer to real‑time.
Market Opportunities:
- Expansion into Ambulatory and Point-of-Care Settings: TBI diagnostics is expanding into ambulatory and point‑of‑care (POC) settings, enabling diagnosis and triage outside traditional hospital emergency departments.
- Expansion into Emerging Markets: The growth in emerging markets is fueled by the escalating number of road accidents, sports-related injuries, and a growing geriatric population, all of which contribute to a higher prevalence of TBIs.
- Wearables, Portable and Point‑of‑Care Diagnostics: Traditional TBI diagnosis, which relies on expensive, bulky, and stationary imaging equipment such as CT and MRI scanners, is inaccessible in emergency, rural, and low-resource environments.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Report Segmentation Analysis
The noninvasive traumatic brain injury diagnostic equipment market is divided into segments to give a clearer view of how it works, its growth potential, and the latest trends. Below is the standard segmentation approach used in industry reports:
By Techniques:
- Electroencephalography (EEG): Electroencephalography (EEG) can provide real-time, functional information about the brain's electrical activity.
- Brain–computer interface (BCI): BCIs are revolutionizing by offering a direct communication pathway between the brain and external devices.
- Eye tracking and near-infrared spectroscopy (NIRS): In addition to the established methods of monitoring brain function, these methods provide further data about the nervous system and brain blood flow.
- Magnetic resonance imaging (MRI): MRI remains a fundamental building block, delivering high-quality, multi-sequence images of brain structure and integrity.
- Magnetoencephalography (MEG): MEG measures the minute magnetic fields generated by the electrical currents of neurons in the brain.
- Transcranial magnetic stimulation (TMS): TMS provides a detailed, noninvasive, and on-the-spot examination of brain functions that can help in the diagnosis of a traumatic brain injury (TBI), where conventional imaging could be ineffective.
- Cerebral metabolic rate of oxygen (CMRO2): CMRO2 directly indicates the brain's metabolic health and oxygen utilization, providing a more accurate evaluation of functional brain injury.
- Intracranial Pressure: ICP monitoring is necessary to detect and control increased intracranial pressure.
- Others: The other segment includes measuring optic nerve sheath diameter (ONSD), transcranial Doppler ultrasound, and infrared optical devices.
By Noninvasive ICP:
- Transcranial Doppler Ultrasonography (TCD): TCD allows continuous, at the patient's bedside, cerebral blood flow measurement, which is an essential aid in the noninvasive diagnosis and the management of traumatic brain injury with a portable, low-cost ultrasound technology.
- Tympanic Membrane Displacement (TMD): Tympanic Membrane Displacement (TMD) is an emerging technology providing a unique approach to estimating ICP
- Optic Nerve Sheath Diameter (ONSD): Optic Nerve Sheath Diameter (ONSD) measurement is known for its effectiveness as a surrogate marker for intracranial pressure (ICP).
- Magnetic Resonance Imaging (MRI) and Computer Tomography (CT): MRI and CT scans remain at the forefront of noninvasive methods for detecting brain injuries. They are tremendously helpful in providing detailed anatomical imaging to make an accurate diagnosis of TBI.
- Fundoscopy and Papilledema: Recent innovations focus on enhancing fundoscopy accuracy and usability for the diagnosis of TBI.
By Device:
- Imaging Devices: Noninvasive traumatic brain injury (TBI) diagnosis greatly benefits from the use of imaging devices such as MRI, CT, and TCD, which facilitate accurate assessment, monitoring, and treatment planning.
- Monitoring Devices: The monitoring devices enable continuous or repeatable assessment of key brain functions without the risks associated with invasive methods.
By End User:
- Hospitals and Clinics
- Diagnostic Centers
- Others
By Geography:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
The regional trends and factors influencing the Noninvasive Traumatic Brain Injury Diagnostic Equipment Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Noninvasive Traumatic Brain Injury Diagnostic Equipment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 1.70 Billion |
| Market Size by 2031 | US$ 3.91 Billion |
| Global CAGR (2025 - 2031) | 12.7% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Techniques
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Players Density: Understanding Its Impact on Business Dynamics
The Noninvasive Traumatic Brain Injury Diagnostic Equipment Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Noninvasive Traumatic Brain Injury Diagnostic Equipment Market top key players overview
Noninvasive traumatic brain injury diagnostic equipment Market Share Analysis by Geography
North America dominates the noninvasive traumatic brain injury diagnostic equipment market with the largest market share. Factors such as the surging number of traumatic brain injuries and a well-established healthcare infrastructure drive market growth. Asia Pacific is expected to grow at the fastest rate in the few years. Emerging markets in South and Central America, the Middle East, and Africa offer untapped opportunities for noninvasive traumatic brain injury diagnostic equipment providers to expand.
The noninvasive traumatic brain injury diagnostic equipment market grows differently in each region. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds a significant portion of the global market
- Key Drivers: Innovative noninvasive diagnostic methods, such as near-infrared spectroscopy (NIRS), and advanced imaging techniques, have revolutionized TBI assessment by offering safer and cost-effective alternatives to traditional methods.
- Trends: Manufacturing advancements and sustainable practices
2. Europe
- Market Share: Substantial share due to the increasing prevalence of brain conditions
- Key Drivers: The region's commitment to improving patient outcomes is evident in developing and adopting advanced diagnostic tools, prioritizing safety, efficiency, and accessibility
- Trends: Regulatory influence on market structure
3. Asia Pacific
- Market Share: Fastest-growing region with rising market shares every year
- Key Drivers: The region, encompassing diverse countries with varying healthcare infrastructures, faces a substantial burden from TBI.
- Trends: Innovations in formulations and delivery
4. South and Central America
- Market Share: A growing market with steady progress
- Key Drivers: Collaborations between international organizations and local governments facilitate the integration of noninvasive diagnostic tools into healthcare systems.
- Trends: Technological Developments in Diagnostic Kits Manufacturing
5. Middle East and Africa
- Market Share: Although small, but growing quickly
- Key Drivers: Governments are implementing specialized workforce training programs in neurotrauma care, while simultaneously investing in the development of pre-hospital infrastructure, including emergency transport systems equipped with mobile diagnostic technologies for TBI.
- Trends: Growth in Diagnostics Products
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is intense due to established players such as GE HealthCare Technologies Inc., Philips, and Elekta AB. Regional and niche providers, such as Natus Medical Inc. and BrainScope Co., Inc., add to the competitive landscape across regions.
This high level of competition urges companies to stand out by offering:
- Advanced products
- Value-added services such as customization and sustainable solutions
- Competitive pricing models
- Compliance with regulatory guidelines
Opportunities and Strategic Moves
- Companies are investing in research and development, which drives innovation in diagnosis technologies. This investment enhances the sensitivity and specificity of the equipment, addressing specific brain health issues in various regions.
- Manufacturers will likely focus on local production to cut costs and strengthen supply chains, especially in high-volume markets such as India.
Other companies analyzed during the course of research:
- Canon Medical Systems Corporation
- Advanced Brain Monitoring, Inc.
- Compumedics Ltd.
- Sense Diagnostics Inc
- NeuroWave Systems, Inc.
- Oculogica Inc.
- Vittamed Corporation
- InfraScan, Inc.
- Neural Analytics Inc.
- CAS Medical Systems, Inc.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market News and Recent Developments
- BrainScope announced the launch of its next-generation deep learning platform. It has developed and commercialized the first FDA-cleared AI/machine learning medical device in the field of neurology.
- GE HealthCare received FDA 510(k) clearance for its innovative SIGNA MAGNUS, a 3.0T high-performance, head-only MRI scanner. This system offers new capabilities for clinical imaging and neuroscience.
Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Report Coverage and Deliverables
The "Noninvasive Traumatic Brain Injury Diagnostic Equipment Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Noninvasive traumatic brain injury diagnostic equipment market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Noninvasive traumatic brain injury diagnostic equipment market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Noninvasive traumatic brain injury diagnostic equipment market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the noninvasive traumatic brain injury diagnostic equipment market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For