Orphan Drugs Market Analysis, Trends, and Forecast by 2031
Coverage: Orphan Drugs Market covers analysis by Active ingredients (Obinutuzumab, Lenalidomide, Brentuximab, Vedotin, Riociguat, Ofatumumab, Nelarabine, Bosutinib, Mannitol, Carglumic acid, Aztreonam, Histamine hydrochloride, Eliglustat, Cabozantinib, Ramucirumab, Decitabine, Defibrotide) ; Disease (Hodgkin lymphoma, Paroxysmal nocturnal hemoglobinuria, Chronic thromboembolic pulmonary hypertension (CTEPH) , Chronic lymphocytic leukemia, Chronic Myeloid Leukaemia, T-cell acute lymphoblastic leukemia (T-ALL) , Philadelphia chromosome positive chronic myelogenous leukaemia (Ph+ CML) , Cystic fibrosis (CF) , acute myeloid leukaemia, Multiple Myeloma, Isovaleric acidaemia, Gaucher disease type 1 (GD1) , Metastatic medullary thyroid carcinoma, Advanced gastric cancer or gastro-esophageal junction adenocarcinoma, Secondary Acute myeloid leukemia (AML) , Severe hepatic venoocclusive disease (VOD) / sinusoidal obstructive syndrome (SOS) ) ; End User (Hospitals, Ambulatory Surgical Centers) , and Geography (North America, Europe, Asia Pacific, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Mar 2026
- Report Code : TIPRE00005799
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
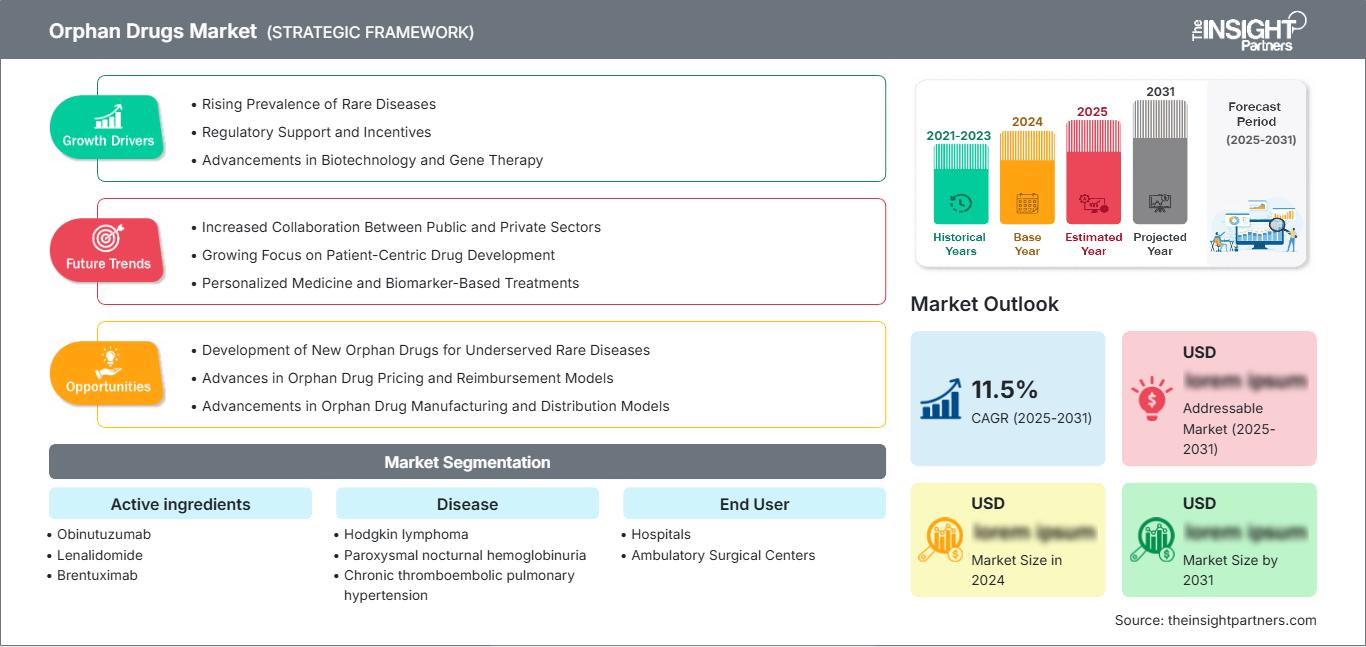
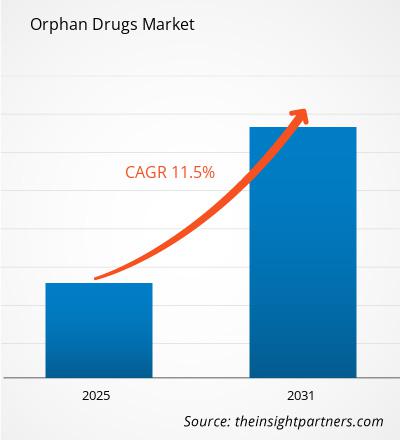
The Orphan Drugs Market size is expected to reach US$ 459.17 Billion by 2031. The market is anticipated to register a CAGR of 10.2% during 2025-2031.
The report is segmented by Active ingredients (Obinutuzumab, Lenalidomide, Brentuximab, Vedotin, Riociguat, Ofatumumab, Nelarabine, Bosutinib, Mannitol, Carglumic acid, Aztreonam, Histamine hydrochloride, Eliglustat, Cabozantinib, Ramucirumab, Decitabine, Defibrotide); Disease (Hodgkin lymphoma, Paroxysmal nocturnal hemoglobinuria, Chronic thromboembolic pulmonary hypertension (CTEPH), Chronic lymphocytic leukemia, Chronic Myeloid Leukaemia, T-cell acute lymphoblastic leukemia (T-ALL), Philadelphia chromosome-positive chronic myelogenous leukaemia (Ph+ CML), Cystic fibrosis (CF), acute myeloid leukaemia, Multiple Myeloma, Isovaleric acidaemia, Gaucher disease type 1 (GD1), Metastatic medullary thyroid carcinoma, Advanced gastric cancer or gastro-esophageal junction adenocarcinoma, Secondary Acute myeloid leukemia (AML), Severe hepatic venoocclusive disease (VOD) / sinusoidal obstructive syndrome (SOS)), and End User (Hospitals, Ambulatory Surgical Centers). The global analysis is broken down at the regional level and for major countries. The market evaluation is presented in US$ for the above segmental analysis.
Purpose of the Report
The report Orphan Drugs Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Orphan Drugs Market Segmentation Active ingredients
- Obinutuzumab
- Lenalidomide
- Brentuximab
- Vedotin
- Riociguat
- Ofatumumab
- Nelarabine
- Bosutinib
- Mannitol
- Carglumic acid
- Aztreonam
- Histamine hydrochloride
- Eliglustat
- Cabozantinib
- Ramucirumab
- Decitabine
- Defibrotide
Disease
- Hodgkin lymphoma
- Paroxysmal nocturnal hemoglobinuria
- Chronic thromboembolic pulmonary hypertension
End User
- Hospitals
- Ambulatory Surgical Centers
Customizee This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONOrphan Drugs Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Orphan Drugs Market Growth Drivers
- Rising Prevalence of Rare Diseases: The increasing global prevalence of rare diseases is a significant growth driver for the Orphan Drugs market. Rare diseases touch few members of the population but together they affect many people across the world. Advanced medical testing helps doctors spot rare diseases earlier while better tests allow them to diagnose these conditions correctly which creates more demand for treatments. Special drugs for rare diseases called orphan drugs help meet the growing medical need. Experts project that rare diseases will keep being diagnosed more often which will drive the need for medical treatments designed for specific conditions. Governments and organizations help treat rare conditions by giving Orphan Drug developers marketing rights and tax benefits. This support, combined with an increasing number of rare disease diagnoses, will continue to drive the growth of the Orphan Drugs market, expanding the market share and ensuring that Orphan Drugs become a larger part of the global healthcare landscape.
- Regulatory Support and Incentives: Governments and healthcare systems need to back up Orphan drug development to make more progress in this market. Governments worldwide support pharmaceutical companies by passing laws that help them develop drugs for rare diseases. Under the Orphan Drug Act in the United States pharmaceutical companies benefit from tax credits market protection and quicker drug approvals to develop rare disease treatments. The European Medicines Agency along with global medical authorities speed up the approval process for orphan drugs through their dedicated programs. These rules lower drug development costs so companies want to make rare disease treatments more often. Market research predicts more orphan drug approvals and market share growth when incentive programs keep running. As more companies leverage these incentives, Orphan Drugs are expected to gain wider acceptance globally, contributing to the overall growth of the market.
- Advancements in Biotechnology and Gene Therapy: The Orphan Drugs market grows because of fast developments in biotechnology and gene therapy research. New technologies help scientists develop biologics and other medical tools like gene therapies and monoclonal antibodies that treat rare diseases well. Gene therapies gained support in the orphan drug industry because they can now treat genetic diseases. Instead of treating symptoms alone, researchers can now use biotechnology tools to find and fix the molecular source of diseases which produces better results. Pharmaceutical companies now invest more in developing orphan drugs because of these new medical breakthroughs. Market research shows biotechnology integration in drug development will grow the market value and market share of Orphan Drugs. The future growth of the market will come from better Orphan Drugs thanks to ongoing development in biotechnology and gene therapy.
Orphan Drugs Market Future Trends
- Increased Collaboration Between Public and Private Sectors: A key trend in the Orphan Drugs market is the growing collaboration between the public and private sectors. Companies in the Orphan Drugs market are working together with both public sector organizations and private businesses more often. Pharmaceutical companies team up with government agencies, non-profits and academic institutions to move Orphan Drugs through development faster. Public-private partnerships share development costs and lower drug research risks for rare diseases. Governments fund research projects and provide money to support orphan drug creation while private companies share their medical know-how and advanced technologies. When private companies and public organizations work together they launch Orphan Drugs faster into the market. Experts expect these partnerships to grow further which will increase Orphan Drug sales while developing more treatments for rare diseases. As the public sector invests in healthcare innovation and the private sector provides the necessary resources, the synergy between the two will drive the overall growth of the Orphan Drugs market.
- Growing Focus on Patient-Centric Drug Development: More drug developers now create medicines with patient needs in mind. The development process now starts with the patient so drugs match their particular health requirements. Pharmaceutical companies need patient experiences and advocacy group feedback at treatment development start to make better medicines for patients. Pharmaceutical companies create Orphan Drugs that help rare disease patients through effective treatments made available to them. Market projections show Orphan Drugs will grow in size because patient-centered development creates medicines that better treat patients and enhance medical results. As pharmaceutical companies continue to focus on developing drugs that address patientsReport Scope
Orphan Drugs Market Regional Insights
The regional trends and factors influencing the Orphan Drugs Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Orphan Drugs Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Orphan Drugs Market Report Scope
By Disease- Hodgkin lymphoma
- Paroxysmal nocturnal hemoglobinuria
- Chronic thromboembolic pulmonary hypertension
- Hospitals
- Ambulatory Surgical Centers
- UK
- Germany
- France
- Russia
- Italy
- Rest of Europe
- China
- India
- Japan
- Australia
- Rest of Asia-Pacific
- Brazil
- Argentina
- Rest of South and Central America
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East and Africa
Report Attribute Details Market size in 2024 US$ XX Billion Market Size by 2031 US$ 459.17 Billion Global CAGR (2025 - 2031) 10.2% Historical Data 2021-2023 Forecast period 2025-2031 Segments Covered By Active ingredients - Obinutuzumab
- Lenalidomide
- Brentuximab
- Vedotin
- Riociguat
- Ofatumumab
- Nelarabine
- Bosutinib
- Mannitol
- Carglumic acid
- Aztreonam
- Histamine hydrochloride
- Eliglustat
- Cabozantinib
- Ramucirumab
- Decitabine
- Defibrotide
Regions and Countries Covered North America - US
- Canada
- Mexico
Market leaders and key company profiles - F. Hoffmann-La Roche Ltd
- Eli Lilly and Company
- Alexion
- CELGENE CORPORATION
- Novartis AG
- Takeda Pharmaceuticals Company Limited
- Biogen
- Bristol-Myers Squibb Company
- Bayer AG
- Sanofi
Orphan Drugs Market Players Density: Understanding Its Impact on Business Dynamics
The Orphan Drugs Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Orphan Drugs Market top key players overview
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Related Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For