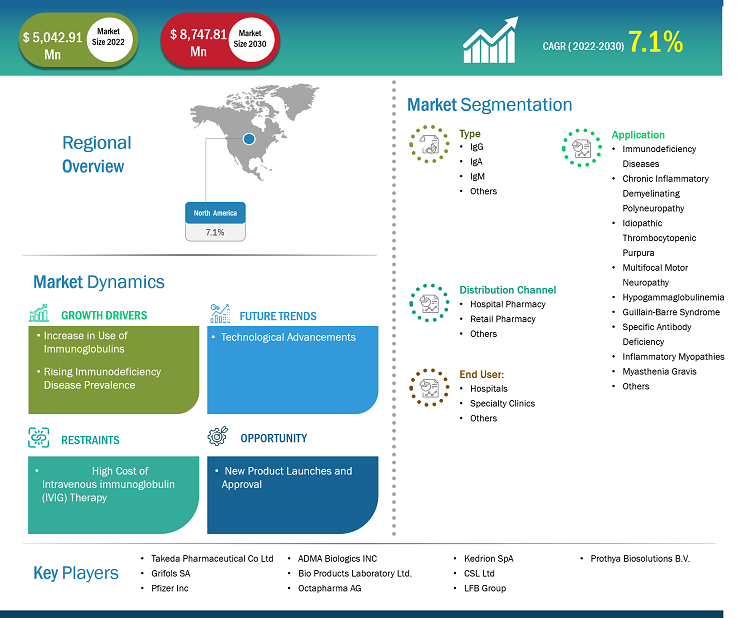
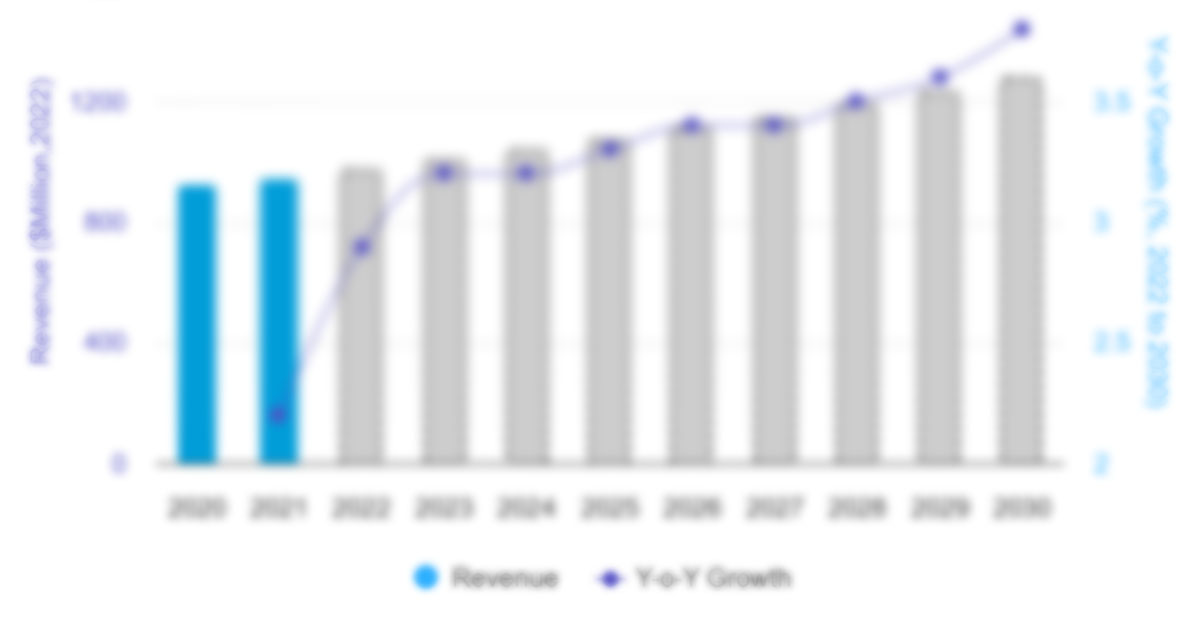
The North America intravenous immunoglobulin market was valued at US$ 5,042.91 million in 2022 and is projected to reach US$ 8,747.81 million by 2030. It is estimated to register a CAGR of 7.1% during 2022–2030.
Market Insights and Analyst View:
Patients with autoimmune diseases are treated with intravenous immunoglobulins. Autoimmune diseases are conditions wherein the immune system accidentally attacks its own tissues or cells. Chronic inflammation, and organ and system damage result from this aberrant immune reaction against healthy cells. Fatigue, joint pain, muscle weakness, skin rashes, and neurological disturbances are a few of the typical signs and symptoms of autoimmune diseases. Symptoms of autoimmune diseases such as Guillain-Barré syndrome (GBS), myasthenia gravis (MS), rheumatoid arthritis (RA), systemic lupus erythematosus (LE), and immune thrombocytopenia (ITP) can be alleviated with the IVIG therapy. Benefits of this therapy include the rapid relief of symptoms and long-lasting effects, leading to improved quality of life among patients.
According to the Intermountain Healthcare, autoimmune and immune-mediated diseases and conditions affect 23.5–50 million Americans. According to the Centers for Disease Control and Prevention, rheumatoid arthritis (RA) is the most prevalent type of autoimmune arthritis, and 1 in 4 adults in the US has arthritis. As per the Myasthenia Gravis Foundation of America, Inc., the prevalence of myasthenia gravis (MG) is estimated at 14–20 per 100,000 of the US population. In Canada, the incidence of MG is estimated to be 23 per 1 million person-years, with a prevalence of 263 per 1 million people, and the numbers have been stable over the past few decades.
With a decrease in the body's capacity to produce T- or B-cells, aging impairs a person's ability to fight off infections and cancerous cells. Immunosenescence, i.e., a weakening of the immune system, is the term used to describe the aging-related changes to the immune system. The elderly population is more prone to immunodeficiency diseases because of immunosenescence. Elderly patients with these diseases are prescribed IVIG therapies. Thus, an upsurge in the geriatric population and the rising prevalence of immunodeficiency disorders propel the growth of the North America intravenous immunoglobulin market
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
North America Intravenous Immunoglobulin Market: Strategic Insights
Market Size Value in US$ 5,042.91 million in 2022 Market Size Value by US$ 8,747.81 million by 2030 Growth rate CAGR of 7.1% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
North America Intravenous Immunoglobulin Market: Strategic Insights

| Market Size Value in | US$ 5,042.91 million in 2022 |
| Market Size Value by | US$ 8,747.81 million by 2030 |
| Growth rate | CAGR of 7.1% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Growth Drivers and Challenges:
Plasma-derived immunoglobulins are used for treating autoimmune and inflammatory disorders, among others. In addition to autoimmune and acute inflammatory conditions, primary immune deficiency disease (PIDD), chronic inflammatory demyelinating polyneuropathy (CIDP), and multifocal motor neuropathy (MMN) are the chronic and acute conditions that are treated with immunoglobulins. Immunoglobulins are also increasingly used to manage infectious diseases, dermatological conditions, rheumatological/nephrological conditions, and heart disease. Thus, the demand for intravenous immunoglobulins is on the rise with the surging use of these antibodies for treating various conditions.
In recent years, various developments have been in the intravenous immunoglobulin market in North America. Market players have been launching new products and seeking regulatory approvals for their offerings. In April 2023, Takeda Pharmaceutical Company Limited, an R&D-driven biopharmaceutical leader, received a supplemental Biologics License Application (sBLA) approval from the US Food and Drug Administration (FDA) to expand the use of HYQVIA to treat primary immunodeficiencies (PI) in children belonging to the age group of 2–16 years. Only HYQVIA's subcutaneous immune globulin (ScIG) infusion allows for monthly administration. In 2022, Health Canada approved HyQvia, a new Immunoglobulin (IG) treatment for Canadians with immune deficiencies. In February 2021, Pfizer Inc. received an sBLA approval for PANZYGA (10% liquid intravenous immunoglobulin preparation) to treat chronic inflammatory demyelinating polyneuropathy (CIDP).
Immunoglobulins are manufactured for infusion so that the finished goods have higher purity levels. Therefore, the cost of producing and purifying intravenous immunoglobulins is high. The total cost of this treatment also varies based on factors like the treatment's length, the disease's diagnosis, and the patient's body weight. For instance, a single IVIG infusion procedure may cost between US$ 100–350 or more. The reported average cost of IVIG therapy in the US is nearly US$ 9,720. If patients receive 4–5 infusions per month, the cost would reach ~US$ 41,796. Thus, the elevated cost of therapy hinders the North America intravenous immunoglobulin market growth
Report Segmentation and Scope:
The North America intravenous immunoglobulin market is divided on the basis of type, application, distribution channel, and end user. Based on type, the North America intravenous immunoglobulin market is segmented into IgG, IgA, IgM, and others. In terms of application, the North America intravenous immunoglobulin marketis segmented into immunodeficiency diseases, chronic inflammatory demyelinating polyneuropathy, idiopathic thrombocytopenic purpura, multifocal motor neuropathy, hypogammaglobulinemia, Guillain-Barre syndrome, specific antibody deficiency, inflammatory myopathies, myasthenia gravis, and others. The North America intravenous immunoglobulin market , by distribution channel, is classified into hospital pharmacy, retail pharmacy, and others. Based on end user, the North America intravenous immunoglobulin marketis divided into specialty clinics, hospitals, and others. Based on country, the North America intravenous immunoglobulin market is divided into the US, Canada, and Mexico.
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
Segmental Analysis:
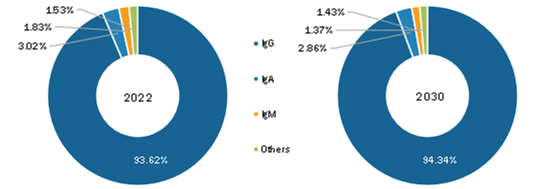
Based on type, the intravenous immunoglobulin market is segmented into IgG, IgA, IgM, and others. IgG (i.e., immunoglobulin G) is the most abundant antibody found in blood, lymph fluid, cerebrospinal fluid, and peritoneal fluid, and it plays a vital role in the humoral immune response. IgG constitutes approximately 75% of total serum antibodies and is equally distributed between intravascular and extravascular serum pools. IgG is the only class of immunoglobulins that can reach fetal circulation by crossing the placental barrier. Human IgG can be divided into four subclasses—IgG1, IgG2, IgG3, and IgG4—based on unique antigenic determinants on their heavy chain constant-region domains and associated biologic functions. Moreover, intravenous immunoglobulin (IVIg) is a replacement therapy and treatment for patients with antibody deficiencies or who suffer from immunodeficiency disorders. For instance, in primary or secondary hypogammaglobulinemia, IVIg replacement therapy protects against infections by providing IgGs in adequate quantities in the blood. IVIg is a blood product prepared from the serum of 1,000-15,000 donors per batch where only the IgG is purified from plasma. IVIg solutions used for treatments contain 95–98% pure IgGs and small amounts of other plasma proteins, including IgAs and IgMs. Thus, the wide application of IgG in treating several categories of disorders will likely complement the growth of the IgG segment during the forecast period.
Intravenous Immunoglobulin Market, by Type – 2022 and 2030
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
Based on the application, the intravenous immunoglobulin market is classified into immunodeficiency diseases, chronic inflammatory demyelinating polyneuropathy, idiopathic thrombocytopenic purpura, multifocal motor neuropathy, hypogammaglobulinemia, Guillain-Barre syndrome, specific antibody deficiency, inflammatory myopathies, myasthenia gravis, and others. In 2022, the immunodeficiency diseases segment held the largest share of the market and is expected to register the highest CAGR during 2022–2030. Immunodeficiency diseases are categorized as primary (congenital) and secondary (acquired) immunodeficiency diseases. Among both, primary immunodeficiency diseases (PIDDs) can be characterized into 400 different types. According to the Journal of Allergy and Clinical Immunology, the prevalence of PIDD in the US is estimated at 1 in 2,000 individuals. Moreover, researchers targeting PIDDs are making great strides toward improving treatment options and enhancing customers' quality of life. IVIg administration is the apparent treatment of choice for humoral primary immunodeficiencies, as these patients cannot mount an effective immune response against pathogens. The US Food and Drug Administration (FDA) approved Privigen, a 10% IVIg liquid, as a replacement therapy against primary immunodeficiency disease (PIDD). In March 2022, ADMA Biologics received FDA approval for its ASCENIV and BIVIgAM immunoglobulin drug products to extend their expiration dates from 24 months to 36 months when stored at 2–8°C. Thus, such regulatory approvals for IVIg products to treat immunodeficiency disorders contribute to the progress of the US intravenous immunoglobulin market for the PIDD segment.
In terms of distribution channels, the intravenous immunoglobulin market is classified into hospital pharmacy, retail pharmacy, and others. In 2022, the hospital pharmacy segment held the largest share of the market, and it is expected to register the highest CAGR during 2022–2030. Hospital pharmacies are among the essential parts of any country's healthcare system. They receive a huge footfall of patients suffering from some type of indication, requiring IVIg replacement therapy. Additionally, all patients need to visit hospitals to receive the scheduled doses of IVIg, which results in the demand for these products in hospital pharmacies. Thus, the hospital pharmacy segment contributes significantly to the intravenous immunoglobulin market.
Based on end user, the intravenous immunoglobulin market is classified into hospitals, specialty clinics, and others. In 2022, the hospitals segment held the largest market share, and it is expected to register the highest CAGR during 2022–2030. Hospitals are complex organizations providing healthcare services with the help of modernized equipment. An increasing number of hospital admissions, coupled with the rising prevalence of immunodeficiency disorders, is projected to drive the growth of the hospital segment in the US intravenous immunoglobulin market during the forecast period. Moreover, a vast demand for advanced hospital settings is experienced in emerging nations for managing a huge patient pool and rising public health concerns.
Hospitals in partnership with companies conduct studies to observe the doses and adverse events related to the therapy and monitor the product's clinical outcomes. Hospitals serve as primary centers for providing immunoglobulin replacement therapies. The perceived advantages of receiving IgRTs in hospitals include greater safety and closer monitoring of patients, and better support from health professionals and experts. Moreover, proper patient-centric care, availability of reimbursement facilities, and other similar benefits provided by hospitals are expected to fuel the US intravenous immunoglobulin market growth for the hospitals segment during the forecast period.
Regional Analysis:
Based on geography, the intravenous immunoglobulin market is divided into the US, Canada, and Mexico. The US is the largest contributor to the market growth in this region, and Canada is expected to record the fastest CAGR during 2022–2030. The rising number of immunodeficiencies and autoimmune diseases in the US is likely to increase the demand for intravenous immunoglobulins. As per the National Institutes of Health, ~23.5 million Americans (over 7% of the population) suffer from an autoimmune disease. Additionally, the US has North America's largest and most commercial pharmaceutical market. It alone holds over 45% of the global pharmaceutical market. Most top global drug manufacturing companies active in biomedical research are headquartered in the US.
In July 2021, the US Food & Drug Administration (FDA) approved Octapharma’s Octagam 10% to treat patients with dermatomyositis, a rare chronic systemic autoimmune disease with a peculiar skin rash and progressive proximal muscle weakness. Further, the FDA approved the investigational new drug (IND) application of Octapharma USA for phase III clinical trial on the efficacy and safety of Octagam 10% [Immune Globulin Intravenous (Human)] therapy in COVID-19 patients with severe disease progression.
Thus, the increasing cases of immunodeficiencies and autoimmune diseases, the flourishing pharmaceutical sector, and surging product approvals are anticipated to boost the US intravenous immunoglobulin market during the forecast period.
Competitive Landscape and Key Companies:
Takeda Pharmaceutical Co Ltd; Grifols SA; Pfizer Inc.; ADMA Biologics, Inc.; Bio Products Laboratory Ltd; Octapharma AG; Kedrion SpA.; CSL Ltd.; LFB Group; and Prothya Biosolutions B.V are a few prominent players operating in the intravenous immunoglobulin market. These companies focus on expanding service offerings to meet the growing consumer demand worldwide. Their global presence allows them to serve a large set of customers, subsequently allowing them to expand their market share.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type, Application, Distribution Channel, End User, and Country

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The growth of the North America intravenous immunoglobulin market is attributed to a few key factors, such as increase in use of immunoglobulins and rising prevalence of immunodeficiency diseases.
Intravenous Immunoglobulin (IVIG) is a therapy treatment for immunocompromised patients. It is made from a collection of immunoglobulins (antibodies) extracted from the plasma of thousands of healthy donors.
U.S. region is dominating the North America intravenous immunoglobulin market in terms of market share and Canada is anticipated to register the highest CAGR during the 2022-2030.
The North America intravenous immunoglobulin market is analyzed based on type, application, distribution channel, and end user. Based on type, the North America intravenous immunoglobulin market is segmented into IgG, IgA, IgM, and Others. Based on application, the North America intravenous immunoglobulin market is classified as immunodeficiency diseases, chronic inflammatory demyelinating polyneuropathy, idiopathic thrombocytopenic purpura, multifocal motor neuropathy, hypogammaglobulinemia, Guillain-Barre syndrome, specific antibody deficiency, inflammatory myopathies, myasthenia gravis, and others. Based on distribution channels, the North America intravenous immunoglobulin market is segmented into hospital pharmacy, retail pharmacy, and others. Based on end users, the North America intravenous immunoglobulin market is classified into hospitals, specialty clinics, and others
The North America intravenous immunoglobulin market majorly consists of the players such as Takeda Pharmaceutical Co Ltd; Grifols SA; Pfizer Inc.; ADMA Biologics, Inc.; Bio Products Laboratory Ltd; Octapharma AG; Kedrion SpA.; CSL Ltd.; LFB Group; and Prothya Biosolutions B.V
1. Introduction
1.1 The Insight Partners Research Report Guidance
1.2 Market Segmentation
2. Executive Summary
2.1 Intravenous Immunoglobulin Market, by Geography
2.2 Key Insights
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. Intravenous Immunoglobulin Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 Global PEST Analysis
5. Intravenous Immunoglobulin Market - Key Industry Dynamics
5.1 Market Drivers:
5.1.1 Increase in Use of Immunoglobulins
5.1.2 Rising Immunodeficiency Disease Prevalence
5.2 Market Restraints
5.2.1 High Cost of Therapy
5.3 Market Opportunities
5.3.1 New Product Launches and Approvals
5.4 Future Trends
5.4.1 Strong Pipeline of IVIG Candidates
5.5 Impact Analysis:
6. Intravenous Immunoglobulin Market – North America Market Analysis
6.1 Intravenous Immunoglobulin Market Revenue (US$ Mn), 2022 – 2030
7. North America Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 – by Type
7.1 Overview
7.2 Intravenous Immunoglobulin Market Revenue Share, by Type, 2022 & 2030 (%)
7.3 IgG
7.3.1 Overview
7.3.2 IgG: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
7.4 IgA
7.4.1 Overview
7.4.2 IgA: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
7.5 IgM
7.5.1 Overview
7.5.2 IgM: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
7.6 Others
7.6.1 Overview
7.6.2 Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8. North America Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 – by Application
8.1 Overview
8.2 Intravenous Immunoglobulin Market Revenue Share, by Application, 2022 & 2030 (%)
8.3 Immunodeficiency Diseases
8.3.1 Overview
8.3.2 Immunodeficiency Diseases: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.4 Chronic Inflammatory Demyelinating Polyneuropathy
8.4.1 Overview
8.4.2 Chronic Inflammatory Demyelinating Polyneuropathy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.5 Idiopathic Thrombocytopenic Purpura
8.5.1 Overview
8.5.2 Idiopathic Thrombocytopenic Purpura: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.6 Multifocal Motor Neuropathy
8.6.1 Overview
8.6.2 Multifocal Motor Neuropathy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.7 Hypogammaglobulinemia
8.7.1 Overview
8.7.2 Hypogammaglobulinemia: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.8 Guillain-Barre Syndrome
8.8.1 Overview
8.8.2 Guillain-Barre Syndrome: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.9 Specific Antibody Deficiency
8.9.1 Overview
8.9.2 Specific Antibody Deficiency: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.10 Inflammatory Myopathies
8.10.1 Overview
8.10.2 Inflammatory Myopathies: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.11 Myasthenia Gravis
8.11.1 Overview
8.11.2 Myasthenia Gravis: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
8.12 Others
8.12.1 Overview
8.12.2 Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
9. North America Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 – by Distribution Channel
9.1 Overview
9.2 Intravenous Immunoglobulin Market Revenue Share, by Route of Administration, 2022 & 2030 (%)
9.3 Hospital Pharmacy
9.3.1 Overview
9.3.2 Hospital Pharmacy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
9.4 Retail Pharmacy
9.4.1 Overview
9.4.2 Retail Pharmacy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
9.5 Others
9.5.1 Overview
9.5.2 Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
10. North America Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 – by End User
10.1 Overview
10.2 Intravenous Immunoglobulin Market Revenue Share, by Species, 2022 & 2030 (%)
10.3 Hospitals
10.3.1 Overview
10.3.2 Hospitals: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
10.4 Specialty Clinics
10.4.1 Overview
10.4.2 Specialty Clinics: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
10.5 Others
10.5.1 Overview
10.5.2 Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
11. North America Intravenous Immunoglobulin Market - Geographical Analysis
11.1 North America Intravenous Immunoglobulin Market, Revenue and Forecast To 2030
11.1.1 Overview
11.1.2 North America Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
11.1.3 North America: Intravenous Immunoglobulin Market, by Type, 2020–2030 (US$ Million)
11.1.4 North America: Intravenous Immunoglobulin Market, by Application, 2020–2030 (US$ Million)
11.1.5 North America: Intravenous Immunoglobulin Market, by Distribution Channel, 2020–2030 (US$ Million)
11.1.6 North America: Intravenous Immunoglobulin Market, by End User, 2020–2030 (US$ Million)
11.1.7 North America Intravenous Immunoglobulin Market, by Country
11.1.7.1 US
11.1.7.1.1 Overview
11.1.7.1.2 US Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
11.1.7.1.3 US: Intravenous Immunoglobulin Market, by Type, 2020–2030 (US$ Million)
11.1.7.1.4 US: Intravenous Immunoglobulin Market, by Application, 2020–2030 (US$ Million)
11.1.7.1.5 US: Intravenous Immunoglobulin Market, by Distribution Channel, 2020–2030 (US$ Million)
11.1.7.1.6 US: Intravenous Immunoglobulin Market, by End User, 2020–2030 (US$ Million)
11.1.7.2 Canada
11.1.7.2.1 Overview
11.1.7.2.2 Canada Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
11.1.7.2.3 Canada: Intravenous Immunoglobulin Market, by Type, 2020–2030 (US$ Million)
11.1.7.2.4 Canada: Intravenous Immunoglobulin Market, by Application, 2020–2030 (US$ Million)
11.1.7.2.5 Canada: Intravenous Immunoglobulin Market, by Distribution Channel, 2020–2030 (US$ Million)
11.1.7.2.6 Canada: Intravenous Immunoglobulin Market, by End User, 2020–2030 (US$ Million)
11.1.7.3 Mexico
11.1.7.3.1 Overview
11.1.7.3.2 Mexico Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
11.1.7.3.3 Mexico: Intravenous Immunoglobulin Market, by Type, 2020–2030 (US$ Million)
11.1.7.3.4 Mexico: Intravenous Immunoglobulin Market, by Application, 2020–2030 (US$ Million)
11.1.7.3.5 Mexico: Intravenous Immunoglobulin Market, by Distribution Channel, 2020–2030 (US$ Million)
11.1.7.3.6 Mexico: Intravenous Immunoglobulin Market, by End User, 2020–2030 (US$ Million)
12. North America Intravenous Immunoglobulin Market – Industry Landscape
12.1 Overview
12.2 Growth Strategies in North America Intravenous Immunoglobulin Market
12.3 Inorganic Growth Strategies
12.3.1 Overview
12.4 Organic Growth Strategies
12.4.1 Overview
13. Company Profiles
13.1 Takeda Pharmaceutical Co Ltd
13.1.1 Key Facts
13.1.2 Business Description
13.1.3 Products and Services
13.1.4 Financial Overview
13.1.5 SWOT Analysis
13.1.6 Key Developments
13.2 Grifols SA
13.2.1 Key Facts
13.2.2 Business Description
13.2.3 Products and Services
13.2.4 Financial Overview
13.2.5 SWOT Analysis
13.2.6 Key Developments
13.3 Pfizer Inc
13.3.1 Key Facts
13.3.2 Business Description
13.3.3 Products and Services
13.3.4 Financial Overview
13.3.5 SWOT Analysis
13.3.6 Key Developments
13.4 ADMA Biologics, Inc.
13.4.1 Key Facts
13.4.2 Business Description
13.4.3 Products and Services
13.4.4 Financial Overview
13.4.5 SWOT Analysis
13.4.6 Key Developments
13.5 Bio Products Laboratory Ltd.
13.5.1 Key Facts
13.5.2 Business Description
13.5.3 Products and Services
13.5.4 Financial Overview
13.5.5 SWOT Analysis
13.5.6 Key Developments
13.6 Octapharma AG
13.6.1 Key Facts
13.6.2 Business Description
13.6.3 Products and Services
13.6.4 Financial Overview
13.6.5 SWOT Analysis
13.6.6 Key Developments
13.7 Kedrion SpA
13.7.1 Key Facts
13.7.2 Business Description
13.7.3 Products and Services
13.7.4 Financial Overview
13.7.5 SWOT Analysis
13.7.6 Key Developments
13.8 CSL Ltd
13.8.1 Key Facts
13.8.2 Business Description
13.8.3 Products and Services
13.8.4 Financial Overview
13.8.5 SWOT Analysis
13.8.6 Key Developments
13.9 LFB Group
13.9.1 Key Facts
13.9.2 Business Description
13.9.3 Products and Services
13.9.4 Financial Overview
13.9.5 SWOT Analysis
13.9.6 Key Developments
13.10 Prothya Biosolutions B.V.
13.10.1 Key Facts
13.10.2 Business Description
13.10.3 Products and Services
13.10.4 Financial Overview
13.10.5 SWOT Analysis
13.10.6 Key Developments
14. Appendix
14.1 About Us
14.2 Glossary of Terms
List of Tables
Table 1. North America Intravenous Immunoglobulin Market Segmentation
Table 2. North America Intravenous Immunoglobulin Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 3. North America Intravenous Immunoglobulin Market, by Application– Revenue and Forecast to 2030 (US$ Million)
Table 4. North America Intravenous Immunoglobulin Market, by Distribution Channel– Revenue and Forecast to 2030 (US$ Million)
Table 5. North America Intravenous Immunoglobulin Market, by End User– Revenue and Forecast to 2030 (US$ Million)
Table 6. US Intravenous Immunoglobulin Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 7. US Intravenous Immunoglobulin Market, by Application– Revenue and Forecast to 2030 (US$ Million)
Table 8. US Intravenous Immunoglobulin Market, by Distribution Channel– Revenue and Forecast to 2030 (US$ Million)
Table 9. US Intravenous Immunoglobulin Market, by End User– Revenue and Forecast to 2030 (US$ Million)
Table 10. Canada Intravenous Immunoglobulin Market, by Type– Revenue and Forecast to 2030 (US$ Million)
Table 11. Canada Intravenous Immunoglobulin Market, by Application– Revenue and Forecast to 2030 (US$ Million)
Table 12. Canada Intravenous Immunoglobulin Market, by Distribution Channel– Revenue and Forecast to 2030 (US$ Million)
Table 13. Canada Intravenous Immunoglobulin Market, by End User– Revenue and Forecast to 2030 (US$ Million)
Table 14. Mexico Intravenous Immunoglobulin Market, by Type– Revenue and Forecast to 2030 (US$ Million)
Table 15. Mexico Intravenous Immunoglobulin Market, by Application– Revenue and Forecast to 2030 (US$ Million)
Table 16. Mexico Intravenous Immunoglobulin Market, by Distribution Channel– Revenue and Forecast to 2030 (US$ Million)
Table 17. Mexico Intravenous Immunoglobulin Market, by End User– Revenue and Forecast to 2030 (US$ Million)
Table 18. Recent Inorganic Growth Strategies in the North America Intravenous Immunoglobulin Market
Table 19. Recent Organic Growth Strategies in North America Intravenous Immunoglobulin Market
Table 20. Glossary of Terms, North America Intravenous Immunoglobulin Market
List of Figures
Figure 1. Intravenous Immunoglobulin Market Segmentation, By Geography
Figure 2. Global - PEST Analysis
Figure 3. Intravenous Immunoglobulin Market - Key Industry Dynamics
Figure 4. Impact Analysis of Drivers and Restraints
Figure 5. Intravenous Immunoglobulin Market Revenue (US$ Mn), 2022 – 2030
Figure 6. Intravenous Immunoglobulin Market Revenue Share, by Type, 2022 & 2030 (%)
Figure 7. IgG: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 8. IgA: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 9. IgM: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 10. Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 11. Intravenous Immunoglobulin Market Revenue Share, by Application, 2022 & 2030 (%)
Figure 12. Immunodeficiency Diseases: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 13. Chronic Inflammatory Demyelinating Polyneuropathy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 14. Idiopathic Thrombocytopenic Purpura: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 15. Multifocal Motor Neuropathy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 16. Hypogammaglobulinemia: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 17. Guillain-Barre Syndrome: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 18. Specific Antibody Deficiency: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 19. Inflammatory Myopathies: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 20. Myasthenia Gravis: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 21. Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 22. Intravenous Immunoglobulin Market Revenue Share, by Route of Administration, 2022 & 2030 (%)
Figure 23. Hospital Pharmacy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 24. Retail Pharmacy: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 25. Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 26. Intravenous Immunoglobulin Market Revenue Share, Species, 2022 & 2030 (%)
Figure 27. Hospitals: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 28. Specialty Clinics: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 29. Others: Intravenous Immunoglobulin Market – Revenue and Forecast to 2030 (US$ Million)
Figure 30. North America: Intravenous Immunoglobulin Market, by Key Country – Revenue (2022) (US$ Million)
Figure 31. North America Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
Figure 32. North America Intravenous Immunoglobulin Market, By Key Countries, 2022 and 2030 (%)
Figure 33. US Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
Figure 34. Canada Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
Figure 35. Mexico Intravenous Immunoglobulin Market Revenue and Forecast to 2030 (US$ Mn)
Figure 36. Growth Strategies in North America Intravenous Immunoglobulin Market
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely - analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You'll receive access to the report within 4-6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we'll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we're happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you're considering, and we'll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report's scope, methodology, customization options, or which license suits you best, we're here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we'll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we'll ensure the issue is resolved quickly so you can access your report without interruption.
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.

Oct 2023
MRI-guided Focused Ultrasound Therapy Market
Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Application (Breast Cancer, Prostate Cancer, Liver Cancer, Pancreatic Cancer, Breast Lifting and Aesthetic Application, Nipple and Areola Preservation, Post Surgical Applications, and Others), End User (Healthcare Facilities, Diagnostic Imaging Centers, and Research Centers), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)

Oct 2023
Gene Therapy CDMO Market
Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type (Drug Development and Manufacturing, Testing and Regulatory Services, and Other Service Types), End User (Pharmaceutical Companies, Biopharmaceutical Companies, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)

Oct 2023
RT-PCR Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Reagents & Consumables, Instruments, and Software & Services), Application (Research Application, Clinical Application, and Forensic Application), End user (Hospitals and Diagnostic Centers, Pharmaceutical and Biotechnology Companies, Research Laboratories and Academic Institutes, Forensic Laboratories, and Clinical Research Organizations)

Oct 2023
dPCR Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Reagents & Consumables, Instruments, and Software & Services), Application (Research Application, Clinical Application, and Forensic Application), End user (Hospitals and Diagnostic Centers, Pharmaceutical and Biotechnology Companies, Research Laboratories and Academic Institutes, Forensic Laboratories, and Clinical Research Organizations)

Oct 2023
Oscillometry Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Device and Accessories), Technology (Impulse Oscillometry, Forced Oscillation Technique, and Hybrid Oscillometry Devices), Application (Asthma, COPD, and Others), End User (Hospitals, Diagnostic Laboratories, and Others)

Oct 2023
Prenatal Testing Services Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Diagnostic Type (Noninvasive and Invasive), Disease (Aneuploidy, Microdeletions, Structural Chromosomal Abnormalities, and Others), End User (Hospitals, Diagnostic Laboratories, Specialty Clinics, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)

Oct 2023
Joint Resurfacing Devices Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Knee, Hip, Shoulder, Ankle, and Others), Material (Metal, Ceramic, and Others), End User (Hospitals, Orthopedic Clinics, Ambulatory Surgical Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South and Central America)

Oct 2023
Embolization Plugs Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Application (Neurology, Peripheral Vascular Disease, Oncology, Urology, and Others), End User (Hospital, Ambulatory Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)


 Get Free Sample For
Get Free Sample For