The North America point of care diagnostics market is expected to grow from US$ 16,612.40 million in 2021 to US$ 38,702.11 million by 2028; it is estimated to grow at a CAGR of 12.8% from 2021 to 2028.
The US, Canada, and Mexico are economies in North America. Growing prevalence of infectious diseases is expected to escalate the market growth. Point-of-care testing (POCT) plays a critical role in diagnosis, treatment, and prevention of infectious diseases. POC testing can be used to detect several major pathogens, including malarial parasites, human immunodeficiency virus (HIV), human papillomavirus (HPV), dengue, Ebola, and Zika viruses; and Mycobacterium tuberculosis (TB bacteria). To narrow down the diagnostic time, companies are developing POC test kits and reagents. The increasing incidence of infectious diseases such as HIV is the major global public health issue. Moreover, healthcare-associated infections (HAIs) such as central line-associated bloodstream infections and catheter-associated urinary tract infections might affect the patients in hospitals and other healthcare facilities. In addition, according to the Office of Disease Prevention and Health Promotion, 1 out of 25 hospitalizations in the US suffers from HAI at any given time. Such increase in prevalence of infectious diseases is expected to create a demand for point of care test kits across the North America.In case of COVID-19, North America is highly affected specially the US. The outbreak of the COVID-19 pandemic situation shown some favorable scenario for players operating in the point of care diagnostics market. Significant population of North America is infected with coronavirus. For instance, the US is a highly affected country in the North American regions. The effect of the corona is mild in adults; however, it is adverse in older people. The infection of corona among older people has resulted in severe complications and has accounted for a good number of deaths in the country. But during the pandemic situation, life science companies engaged in development of novel drugs for the treatment of life threaten diseases. In addition, increasing demand for rapid testing equipment is also playing a prominent role in the growth of the North America point of care diagnostics market. Furthermore, due to the rising numbers of COVID-19 population has positive impact on the adoption of point of care diagnostic kits. This adoption is boosting new product development and launches in the market. For instance, in July 2020, Eurofins U.S. Clinical Diagnostics has announced the availability of its pooled PCR test to detect SARS-CoV-2, which would substantially lower the cost per PCR test for clients. Moreover, in March 2020, Abbott received FDA approval for its molecular point-of-care test for the diagnosis of COVID-19. The test is capable to deliver positive results in five minutes and negative result in less than fifteen minutes. Such product launches are expected to accelerate the growth of the North America point of care diagnostics market.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the North America point of care diagnostics market. The North America point of care diagnostics market is expected to grow at a good CAGR during the forecast period.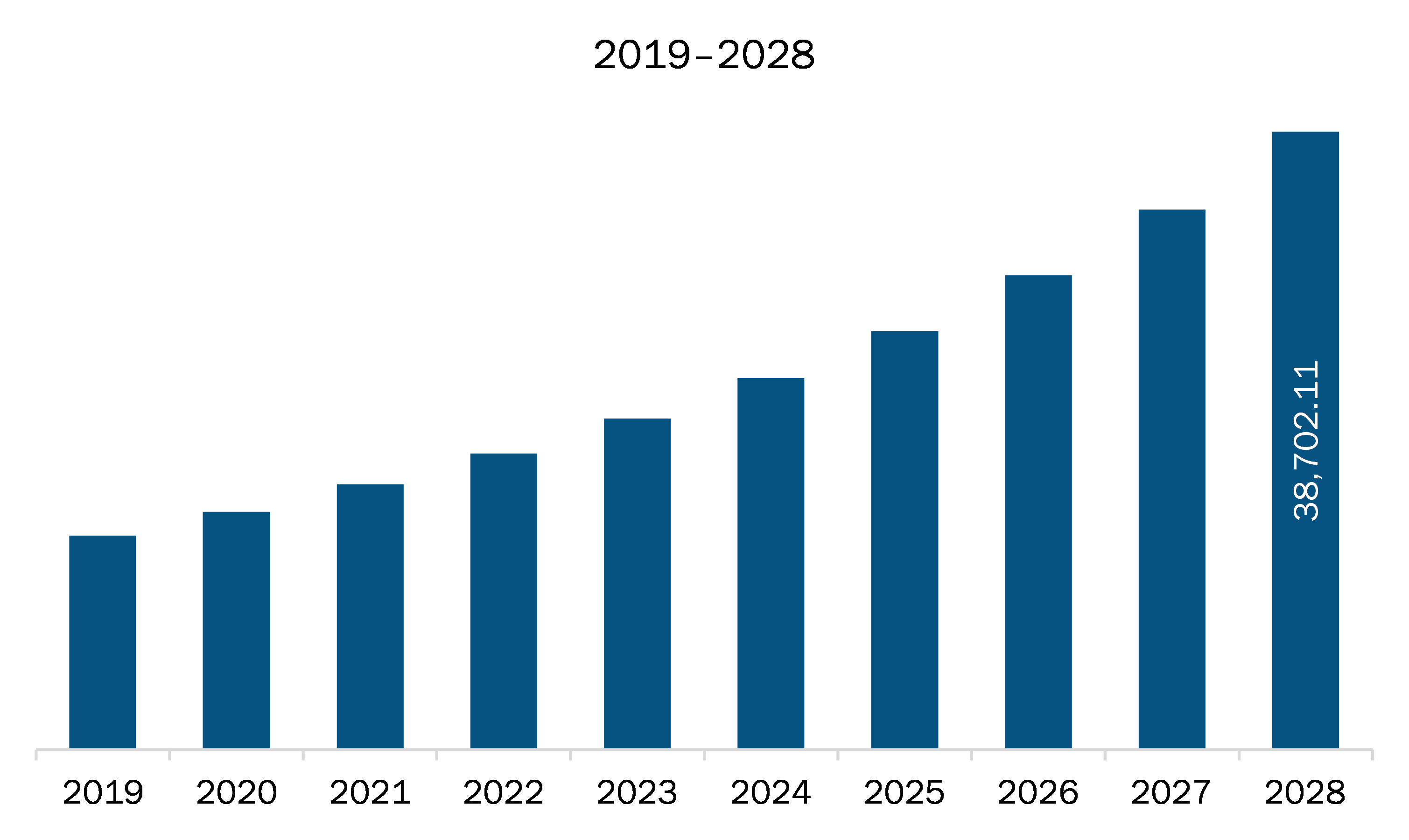
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
North America Point of Care Diagnostics Market Segmentation
North America Point of Care Diagnostics Market – By Product
- Glucose Monitoring Products
- Infectious Disease Testing Products
- HIV Testing
- Influenza Testing
- Sexually Transmitted Disease (STD) Testing
- Hepatitis C Testing
- Healthcare-Associated Infection (HAI) Testing
- Tropical Disease Testing
- Respiratory Infection Testing
- Other Infectious Disease Testing
- Cardiometabolic Testing Products
- Pregnancy and Fertility Testing Products
- Coagulation Testing Products
- Tumor/Cancer Marker Testing Products
- Cholesterol Testing Products
- Urinalysis Testing Products
- Hematology Testing Products
- Other POC Products
North America Point of Care Diagnostics Market – By Prescription Mode
- Prescription-Based Testing
- OTC Testing
North America Point of Care Diagnostics Market – By End User
- Professional Diagnostic Centers
- Hospitals
- Clinical Laboratories
- Outpatient Healthcare
- Ambulatory Care Settings
- Home Care
- Research Laboratories
- Others
North America Point of Care Diagnostics Market, by Country
- US
- Canada
- Mexico
North America Point of Care Diagnostics Market -Companies Mentioned
- Abbott
- BD
- bioMerieux SA
- BIO-RAD LABORATORIES INC.
- Danaher
- F. HOFFMANN-LA ROCHE LTD.
- Johnson and Johnson Services, Inc.
- Nova Biomedical
- Polymer Technology Systems, Inc. (PTS)
- Siemens AG

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product, Prescription Mode, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
US, Canada
TABLE OF CONTENTS
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 North America Point of Care Diagnostics Market – By Product
1.3.2 North America Point of Care Diagnostics Market – By Prescription Mode
1.3.3 North America Point of Care Diagnostics Market – By End User
1.3.4 North America Point of Care Diagnostics Market – By Country
2. Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. North America Point of Care Diagnostics Market – Market Landscape
4.1 Overview
4.2 North America PEST Analysis
5. North America Point of Care Diagnostics Market – Key Industry Dynamics
5.1 Key Market Drivers
5.1.1 Growing Prevalence of Infectious Diseases
5.1.2 Increasing Product Launches and Product Developments
5.1.3 Rising Number of CLIA-waived POC tests
5.2 Market Restraints
5.2.1 Product Recalls in Market
5.3 Market Opportunities
5.3.1 Burgeoning Number of Conferences and Events
5.4 Future Trends
5.4.1 Escalating Use of Home-Based POC Devices
5.5 Impact Analysis
6. Point of Care Diagnostics Market – North America Analysis
6.1 North America Point of Care Diagnostics Market Revenue Forecast and Analysis
7. North America Point of Care Diagnostics Market Analysis– by Product
7.1 Overview
7.2 North America Point of Care Diagnostics Market, By Product 2020 & 2028 (%)
7.3 Glucose Monitoring
7.3.1 Overview
7.3.2 Glucose Monitoring Market Revenue and Forecasts to 2028 (US$ Mn)
7.4 Infectious Disease Testing
7.4.1 Overview
7.4.2 Infectious Disease Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.3 HIV Testing
7.4.3.1 Overview
7.4.3.2 HIV Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.4 Influenza Testing
7.4.4.1 Overview
7.4.4.2 Influenza Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.5 Sexually Transmitted Disease (STD) Testing
7.4.5.1 Overview
7.4.5.2 Sexually Transmitted Disease (STD) Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.6 Hepatitis C Testing
7.4.6.1 Overview
7.4.6.2 Hepatitis C Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.7 Healthcare-Associated Infection (HAI) Testing
7.4.7.1 Overview
7.4.7.2 Healthcare-Associated Infection (HAI) Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.8 Tropical Disease Testing
7.4.8.1 Overview
7.4.8.2 Tropical Disease Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.9 Respiratory Infection Testing
7.4.9.1 Overview
7.4.9.2 Respiratory Infection Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.4.10 Other Infectious Disease
7.4.10.1 Overview
7.4.10.2 Others Infection Disease Market Revenue and Forecasts to 2028 (US$ Mn)
7.5 Cardiometabolic Testing
7.5.1 Overview
7.5.2 Cardiometabolic Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.6 Pregnancy and Fertility Testing
7.6.1 Overview
7.6.2 Pregnancy and Fertility Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.7 Coagulation Testing
7.7.1 Overview
7.7.2 Coagulation Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.8 Tumor/Cancer Marker Testing
7.8.1 Overview
7.8.2 Tumor/Cancer Marker Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.9 Cholesterol Testing
7.9.1 Overview
7.9.2 Cholesterol Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.10 Urinalysis Testing
7.10.1 Overview
7.10.2 Urinalysis Testing Market Revenue and Forecasts to 2028 (US$ Mn)
7.11 Haematology Testing
7.11.1 Overview
7.11.2 Haematology Testing Revenue and Forecasts to 2028 (US$ Mn)
7.12 Other POC
7.12.1 Overview
7.12.2 Other POC Market Revenue and Forecasts to 2028 (US$ Mn)
8. North America Point of Care Diagnostics Market Analysis– by Prescription Mode
8.1 Overview
8.2 North America Point of Care Diagnostics Market, By Prescription Mode 2020 & 2028 (%)
8.3 Prescription-Based Testing Market
8.3.1 Overview
8.3.2 Prescription-Based Testing Market Revenue and Forecasts to 2028 (US$ Mn)
8.4 OTC Testing Market
8.4.1 Overview
8.4.2 OTC Testing Market Revenue and Forecasts to 2028 (US$ Mn)
9. North America Point of Care Diagnostics Market Analysis– by End User
9.1 Overview
9.2 North America Point of Care Diagnostics Market, By End User 2020 & 2028 (%)
9.3 Professional Diagnostic Centers
9.3.1 Overview
9.3.2 Professional Diagnostic Centers Market Revenue and Forecasts to 2028 (US$ Mn)
9.3.3 Hospitals
9.3.3.1 Overview
9.3.3.2 Hospitals Market Revenue and Forecasts to 2028 (US$ Mn)
9.3.4 Clinical Laboratories
9.3.4.1 Overview
9.3.4.2 Clinical Laboratories Market Revenue and Forecasts to 2028 (US$ Mn)
9.3.5 Outpatient Healthcare
9.3.5.1 Overview
9.3.5.2 Outpatient Healthcare Market Revenue and Forecasts to 2028 (US$ Mn)
9.3.6 Ambulatory Care Settings
9.3.6.1 Overview
9.3.6.2 Ambulatory Care Settings Market Revenue and Forecasts to 2028 (US$ Mn)
9.4 Home Care
9.4.1 Overview
9.4.2 Home Care Market Revenue and Forecasts to 2028 (US$ Mn)
9.5 Research Laboratories
9.5.1 Overview
9.5.2 Research Laboratories Market Revenue and Forecasts to 2028 (US$ Mn)
9.6 Others
9.6.1 Overview
9.6.2 Others Market Revenue and Forecasts to 2028 (US$ Mn)
10. North America Point of Care Diagnostics Market – Country Analysis
10.1 North America: Point of Care Diagnostics Market
10.1.1 Overview
10.1.2 North America: Point of Care Diagnostics Market, By Country, 2020 & 2028 (%)
10.1.3 US: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
10.1.3.1 US: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
10.1.3.2 US: Point of Care Diagnostics Market, By Product, 2019-2028 (USD Million)
10.1.3.2.1 US: Infectious Disease Testing Products Market, By Infectious Disease Testing, 2019-2028 (USD Million)
10.1.3.3 US: Point of Care Diagnostics Market, By Prescription Mode, 2019-2028 (USD Million)
10.1.3.4 US: Point of Care Diagnostics Market, By End User, 2019-2028 (USD Million)
10.1.3.4.1 US: Professional Diagnostic Centres Market, By Professional Diagnostic Centres, 2019-2028 (USD Million)
10.1.4 Canada: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
10.1.4.1 Canada: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
10.1.4.2 Canada: Point of Care Diagnostics Market, By Product, 2019-2028 (USD Million)
10.1.4.2.1 Canada: Infectious Disease Testing Products Market, By Infectious Disease Testing, 2019-2028 (USD Million)
10.1.4.3 Canada: Point of Care Diagnostics Market, By Prescription Mode, 2019-2028 (USD Million)
10.1.4.4 Canada: Point of Care Diagnostics Market, By End User, 2019-2028 (USD Million)
10.1.4.4.1 Canada: Professional Diagnostic Centres Market, By Professional Diagnostic Centres, 2019-2028 (USD Million)
10.1.5 Mexico: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
10.1.5.1 Mexico: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
10.1.5.2 Mexico: Point of Care Diagnostics Market, By Product, 2019-2028 (USD Million)
10.1.5.2.1 Mexico: Infectious Disease Testing Products Market, By Infectious Disease Testing, 2019-2028 (USD Million)
10.1.5.3 Mexico: Point of Care Diagnostics Market, By Prescription Mode, 2019-2028 (USD Million)
10.1.5.4 Mexico: Point of Care Diagnostics Market, By End User, 2019-2028 (USD Million)
10.1.5.4.1 Mexico: Professional Diagnostic Centres Market, By Professional Diagnostic Centres, 2019-2028 (USD Million)
11. Impact of COVID-19 Pandemic on North America Point of Care Diagnostics Market
11.1 North America: Impact Assessment of COVID-19 Pandemic
12. Industry Landscape
12.1 Overview
12.2 Organic Growth Strategies
12.2.1 Overview
12.3 Inorganic Growth Strategies
12.3.1 Overview
13. Company Profiles
13.1 F. HOFFMANN-LA ROCHE LTD.
13.1.1 Key Facts
13.1.2 Business Description
13.1.3 Products and Services
13.1.4 Financial Overview
13.1.5 SWOT Analysis
13.1.6 Key Developments
13.2 Danaher
13.2.1 Key Facts
13.2.2 Business Description
13.2.3 Products and Services
13.2.4 Financial Overview
13.2.5 SWOT Analysis
13.2.6 Key Developments
13.3 BIO-RAD LABORATORIES INC.
13.3.1 Key Facts
13.3.2 Business Description
13.3.3 Products and Services
13.3.4 Financial Overview
13.3.5 SWOT Analysis
13.3.6 Key Developments
13.4 Abbott
13.4.1 Key Facts
13.4.2 Business Description
13.4.3 Products and Services
13.4.4 Financial Overview
13.4.5 SWOT Analysis
13.4.6 Key Developments
13.5 Johnson and Johnson Services, Inc.
13.5.1 Key Facts
13.5.2 Business Description
13.5.3 Products and Services
13.5.4 Financial Overview
13.5.5 SWOT Analysis
13.5.6 Key Developments
13.6 Siemens AG
13.6.1 Key Facts
13.6.2 Business Description
13.6.3 Products and Services
13.6.4 Financial Overview
13.6.5 SWOT Analysis
13.6.6 Key Developments
13.7 BD
13.7.1 Key Facts
13.7.2 Business Description
13.7.3 Products and Services
13.7.4 Financial Overview
13.7.5 SWOT Analysis
13.7.6 Key Developments
13.8 bioMerieux SA
13.8.1 Key Facts
13.8.2 Business Description
13.8.3 Products and Services
13.8.4 Financial Overview
13.8.5 SWOT Analysis
13.8.6 Key Developments
13.9 Polymer Technology Systems, Inc. (PTS)
13.9.1 Key Facts
13.9.2 Business Description
13.9.3 Products and Services
13.9.4 Financial Overview
13.9.5 SWOT Analysis
13.9.6 Key Developments
13.10 Nova Biomedical
13.10.1 Key Facts
13.10.2 Business Description
13.10.3 Products and Services
13.10.4 Financial Overview
13.10.5 SWOT Analysis
13.10.6 Key Developments
14. Appendix
14.1 About the Insight Partners
14.2 Glossary of Terms
LIST OF TABLES
Table 1. North America Point of Care Diagnostics Market – Revenue and Forecast to 2028 (US$ Million)
Table 2. US: Point of Care Diagnostics Market, By Product - Revenue and Forecast to 2028 (USD Million)
Table 3. US: Infectious Disease Testing Products Market, By Infectious Disease Testing- Revenue and Forecast to 2028 (USD Million)
Table 4. US: Point of Care Diagnostics Market, By Prescription Mode - Revenue and Forecast to 2028 (USD Million)
Table 5. US: Point of Care Diagnostics Market, By End User - Revenue and Forecast to 2028 (USD Million)
Table 6. US: Professional Diagnostic Centers Market, By Professional Diagnostic Centers- Revenue and Forecast to 2028 (USD Million)
Table 7. Canada: Point of Care Diagnostics Market, By Product - Revenue and Forecast to 2028 (USD Million)
Table 8. Canada: Infectious Disease Testing Products Market, By Infectious Disease Testing- Revenue and Forecast to 2028 (USD Million)
Table 9. Canada: Point of Care Diagnostics Market, By Prescription Mode - Revenue and Forecast to 2028 (USD Million)
Table 10. Canada: Point of Care Diagnostics Market, By End User - Revenue and Forecast to 2028 (USD Million)
Table 11. Canada: Professional Diagnostic Centers Market, By Professional Diagnostic Centers- Revenue and Forecast to 2028 (USD Million)
Table 12. Mexico: Point of Care Diagnostics Market, By Product - Revenue and Forecast to 2028 (USD Million)
Table 13. Mexico: Infectious Disease Testing Products Market, By Infectious Disease Testing- Revenue and Forecast to 2028 (USD Million)
Table 14. Mexico: Point of Care Diagnostics Market, By Prescription Mode - Revenue and Forecast to 2028 (USD Million)
Table 15. Mexico: Point of Care Diagnostics Market, By End User - Revenue and Forecast to 2028 (USD Million)
Table 16. Mexico: Professional Diagnostic Centers Market, By Professional Diagnostic Centers- Revenue and Forecast to 2028 (USD Million)
Table 17. Recent Organic Developments the Point of Care Diagnostics Market
Table 18. Recent Inorganic Developments in the Point of Care Diagnostics Market
Table 19. Glossary of Terms
LIST OF FIGURES
Figure 1. North America Point of Care Diagnostics Market Segmentation
Figure 2. North America Point of Care Diagnostics Market Segmentation, By Country
Figure 3. North America Point of Care Diagnostics Market Overview
Figure 4. Prescription-Based Testing Segment Held Largest Share of Point of North America Care Diagnostics Market
Figure 5. US to Show Significant Growth During Forecast Period
Figure 6. North America: PEST Analysis
Figure 7. Impact Analysis of Driver and Restraints Pertaining to North America Point of Care Diagnostics Market
Figure 8. North America Point of Care Diagnostics Market – Revenue Forecast and Analysis
Figure 9. North America Point of Care Diagnostics Market, by Products 2020 & 2028 (%)
Figure 10. North America Glucose Monitoring Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 11. North America Infectious Disease Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 12. North America HIV Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 13. North America Influenza Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 14. North America Sexually Transmitted Disease (STD) Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 15. North America Hepatitis C Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 16. North America Healthcare-Associated Infection (HAI) Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 17. North America Tropical Disease Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 18. North America Respiratory Infection Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 19. North America Others Infection Disease Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 20. North America Cardiometabolic Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 21. North America Pregnancy and Fertility Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 22. North America Coagulation Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 23. North America Tumor/Cancer Marker Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 24. North America Cholesterol Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 25. North America Urinalysis Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 26. North America Haematology Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 27. North America Other POC Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 28. North America Point of Care Diagnostics Market, by Prescription Mode 2020 & 2028 (%)
Figure 29. North America Prescription-Based Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 30. North America OTC Testing Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 31. North America Point of Care Diagnostics Market, by End User 2020 & 2028 (%)
Figure 32. North America Professional Diagnostic Centers Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 33. North America Hospitals Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 34. North America Clinical Laboratories Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 35. North America Outpatient Healthcare Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 36. North America Ambulatory Care Settings Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 37. North America Home Care Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 38. North America Research Laboratories Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 39. North America Others Market Revenue and Forecasts to 2028 (US$ Mn)
Figure 40. North America: Point of Care Diagnostics Market, by Key Country- Revenue (2020) (USD Million)
Figure 41. North America: Point of Care Diagnostics Market, By Country, 2020 & 2028 (%)
Figure 42. US: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
Figure 43. Canada: Point of Care Diagnostics Market - Revenue and Forecast to 2028 (USD Million)
Figure 44. Mexico Point of care diagnostics Market Revenue and Forecast to 2028 (US$ Mn)
Figure 45. Impact of COVID-19 Pandemic in North American Country Markets
- Abbott
- BD
- bioMerieux SA
- BIO-RAD LABORATORIES INC.
- Danaher
- F. HOFFMANN-LA ROCHE LTD.
- Johnson and Johnson Services, Inc.
- Nova Biomedical
- Polymer Technology Systems, Inc. (PTS)
- Siemens AG
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.

Jul 2021
Wheelchair and Mobility Aids Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Wheelchairs (Manual, Powered, Power Assisted, and Accessories), Mobility Scooters, Walking Aids, and Others), Application (Neurologically Impaired, Handicap Patients, and Other Applications), End User (Homecare, Hospitals and Clinics, Rehabilitation Centers, and Others), and Distribution Channel (Online and Offline)

Jul 2021
Cystoscopy and Ureteroscopy Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type [Cystoscope (Rigid and Flexible), and Ureteroscope (Flexible and Semi-Flexible)], Procedure (Stone Management, Bladder Cancer, Benign Prostatic Hyperplasia, and Others), Technology (Fiberoptic and Video), Usage Type (Reusable and Single Use), End User (Hospitals, Ambulatory Surgical Centers and Specialty Clinics), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, South and Central America)

Jul 2021
Cancer Drugs Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Therapy Type (Chemotherapy, Targeted Therapy, Radiation Therapy, Hormone Therapy, and Other Therapy Types), By Indications (Blood Cancer, Lung Cancer, Breast Cancer, Colorectal Cancer, Prostate Cancer, Stomach Cancer, Cervical Cancer, Liver and Intrahepatic Bile Ducts Cancer, Thyroid Cancer, and Other Indications), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Stores), and Geography

Jul 2021
Walking Aids Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Canes, Crutches, Walkers, Rollators, and Others), Age Group (Adults and Pediatric), Application (Neurologically Impaired, Handicap Patients, and Others), End User (Homecare, Hospitals and Clinics, Rehabilitation Centers, and Others), and Distribution Channel (Online and Offline)

Jul 2021
Infectious Disease In vitro Diagnostics Market
Size and Forecast (2021-2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: Application (HIV or AIDS, Tuberculosis, Hepatitis B and C, Malaria, and Others), End User (Hospitals and Clinics, Diagnostic Laboratories, Blood Bank, and Others), and Geography

Jul 2021
Donor Blood Components Market
Size and Forecast (2021–2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: Blood Component (Red Blood Cells, White Blood Cells, Platelets, Plasma, and Others), Indication (Immunosuppression and Oncology), Application (Anemia Management, Thrombocytopenia Treatment, Coagulopathy Correction, Supportive Care During Chemotherapy, Surgery, and Others), Age Group (Adult and Child), End User (Hospitals, Oncology Centers, Transplant Centers, Ambulatory Surgical Centers, and Others), and Geography

Jul 2021
Aesthetic Injectable Devices Market
Size and Forecast (2021-2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: Product (Dermal Filler, Botulinum Toxin and Others), Dermal Fillers (Calcium Hydroxylapatite, Hyaluronic Acid, Collagen, Poly-L-Lactic Acid, Polymethylmethacrylate, Fat Fillers, and Others), Device Type (Microcannulas, Pre-Filled Syringes, Mesotherapy Guns, and Others), Application (Facial Line Correction, Face Lift, Lip Treatment, Anti-Ageing and Wrinkle Treatment, Acne and Scar Treatment, Stretch Marks, and Others), Age Group (Up to 30, 31-40, 41-50, 51-60, and More Than 60), Gender (Female and Male), End User (Medical Spas, Dermatology Clinics, Hospitals, and Others), and Geography

Jul 2021
ECG Monitoring Equipment Market
Size and Forecast (2021-2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Resting ECG, Stress ECG, Holter Monitors, and Cardiopulmonary Stress Testing Monitors), Lead Type (12-Lead ECG, 3–6 Lead ECG, and Single Lead), Technology [Portable (Wired) ECG System and Wireless ECG System], End User (Hospital and Clinics, Ambulatory Surgical Centers, Cardiac Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)


 Get Free Sample For
Get Free Sample For