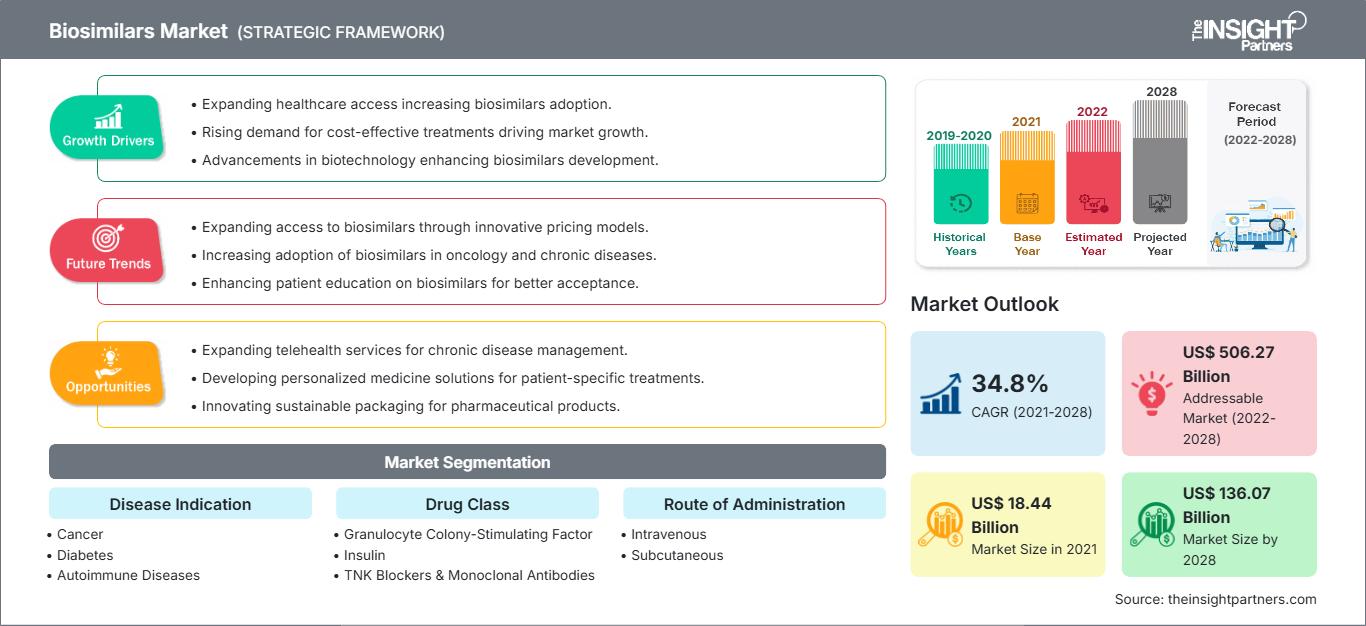
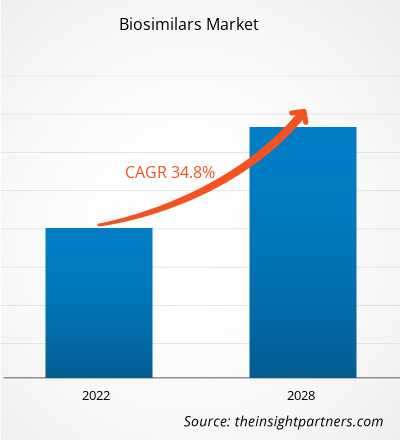
[研究报告]生物仿制药市场规模预计将从2021年的184.3589亿美元增长到2028年的1360.6953亿美元;预计2022年至2028年的复合年增长率为34.8%。
分析师观点
推动生物仿制药市场增长的主要因素是癌症等慢性疾病发病率的上升。日益加重的癌症负担和由此导致的死亡人数的增加,使得人们对负担得起的治疗方案产生了需求,从而推动了生物仿制药市场的增长。主要市场参与者还通过各种战略活动(例如产品发布、并购)预测了预测期内的市场增长。强直性脊柱炎和类风湿性关节炎等自身免疫性疾病的患病率上升推动了生物仿制药市场规模的增长。例如,根据2020年发表在《斯堪的纳维亚风湿病学杂志》上的一篇题为《西班牙强直性脊柱炎患病率》的论文,约7.3%的人口在强直性脊柱炎筛查中呈阳性。生物类似药,例如英夫利昔单抗-axxq(Avsola)、英夫利昔单抗-qbtx(Ixifi)、英夫利昔单抗-dyyb(Inflectra)和英夫利昔单抗-abda(Renflexis),用于治疗慢性关节炎疼痛。
市场概览
生物类似药是指与另一种已获批准的生物药(“参比药”)高度相似的生物药。生物类似药的审批遵循适用于所有生物药的相同药品质量、安全性和有效性标准。生物类似药是许多疾病的安全有效治疗选择,例如慢性皮肤和肠道疾病(如牛皮癣、肠易激综合征、克罗恩病和结肠炎)、关节炎、肾脏疾病和癌症。生物类似药可以以更低的成本增加人们获得救命药物的渠道。生物仿制药市场的主要驱动力是慢性病发病率的上升。
自定义此报告以满足您的要求
您将免费获得任何报告的定制,包括本报告的部分内容,或国家级分析、Excel 数据包,以及为初创企业和大学提供超值优惠和折扣
生物仿制药市场: 战略洞察
-
获取本报告的主要市场趋势。这个免费样本将包括数据分析,从市场趋势到估计和预测。
市场驱动因素
生物仿制药审批不断增加,推动全球生物仿制药市场增长
美国食品药品监督管理局 (FDA) 负责批准生物仿制药产品,并提供将安全有效的生物仿制药推向市场所需的科学和监管建议。生物类似药的批准可以增加药物选择的数量,并可能降低成本,从而改善患者护理。
下表列出了一些近期批准的生物类似药。
生物类似药名称 |
批准日期 |
参考产品 |
Alymsys(贝伐单抗-maly) |
2022 年 4 月 |
Avastin(贝伐单抗) |
Cimerli(雷珠单抗-eqrn) |
2022 年 8 月 |
Lucentis (雷珠单抗) |
Fylnetra(聚乙二醇非格司亭-pbbk) |
2022 年 5 月 |
Neulasta(聚乙二醇非格司亭) |
Stimufend (pegfilgrastim-fpgk) |
2022 年 9 月 |
Neulasta(pegfilgrastim) |
Vegzelma(贝伐珠单抗-adcd) |
9 月2022 |
阿瓦斯汀(贝伐珠单抗) |
Idacio (阿达木单抗-aacf) |
2022 年 12 月 |
Humira(阿达木单抗) |
Byooviz(雷珠单抗-nuna) |
9 月2021 |
Lucentis(雷珠单抗) |
Rezvoglar(甘精胰岛素-aglr) |
2021 年 12 月 |
来得时(胰岛素甘精胰岛素) |
Semglee(甘精胰岛素-yfgn) |
七月2021年 |
来得时(甘精胰岛素) |
Yusimry(阿达木单抗-aqvh) |
2021年12月 |
Humira (阿达木单抗) |
Hulio(阿达木单抗-fkjp) |
2020 年 7 月 |
修美乐(阿达木单抗) |
Riabni (利妥昔单抗-arrx) |
2020 年 12 月 |
Rituxan (利妥昔单抗) |
Nyvepria (pegfilgrastim-apgf) |
2020年6月 |
Neulasta (pegfilgrastim) |
因此,生物仿制药的获批数量不断增加,推动了生物仿制药市场的增长。
分部分析
根据疾病适应症,生物仿制药市场细分为癌症、糖尿病、自身免疫性疾病和其他疾病适应症。癌症领域在2021年占据了最大的市场份额,而自身免疫性疾病预计在预测期内(2022年至2028年)的复合年增长率最高,为36.1%。根据药物类别,生物仿制药市场细分为粒细胞集落刺激因子、人类生长激素、胰岛素、TNF 阻滞剂和单克隆抗体以及其他(骨质疏松症等)。粒细胞集落刺激因子药物类别在 2021 年占据了最大的市场份额。此外,预计其他药物类别在预测期内将以最高的复合年增长率增长。根据应用,全球生物仿制药市场分为静脉注射、皮下注射和其他应用。静脉注射部分在 2021 年占据了最大的市场份额,预计在预测期内将以最高的复合年增长率增长。按最终用户划分,生物仿制药市场分为医院、专科诊所、家庭护理和其他最终用户。 2021 年,医院部门占据了最大的市场份额,而预测期内(2022 年至 2028 年),家庭护理部门预计将实现最高的市场复合年增长率,达到 36.6%。
区域分析
2021 年,北美生物仿制药市场价值为 54.7984 亿美元,预计到 2028 年将达到 477.468 亿美元;预计预测期内的复合年增长率为 37.3%。北美生物仿制药市场分为美国、加拿大和墨西哥。2019 年,美国占据了北美生物仿制药市场的最大份额。糖尿病、不孕不育发病率不断上升,生物仿制药市场的产品开发也在不断增加。据美国国立卫生研究院自身免疫性疾病协调委员会统计,2019 年,超过 2400 万美国人患有自身免疫性疾病。800 万人体内有自身抗体,这种血液分子可以指示一个人罹患自身免疫性疾病的风险。由于未知原因,自身免疫性疾病正在影响越来越多的人。据美国国家环境健康科学研究所 (NIEHS) 临床研究部门统计,2020 年,抗核抗体 (ANA) 的患病率显著增加,ANA 是美国最常见的自身免疫生物标志物。这项研究首次评估了美国人口代表性样本中 ANA 随时间的变化。研究对象包括男性、非西班牙裔白人、50 岁以上的成年人和青少年。在美国,生物仿制药用于治疗癌症、肾脏疾病、糖尿病以及其他自身免疫性疾病,如类风湿性关节炎和克罗恩病。据康德乐公司(Cardinal Health)称,美国食品药品监督管理局(FDA)已批准33种生物类似药,其中21种已上市。在已上市的21种生物类似药中,17种用于治疗癌症,3种用于治疗自身免疫性疾病,1种用于治疗糖尿病。
生物制剂是美国最昂贵的药物,每位患者每年的医疗费用高达数万美元。预计生物类似药的价格将比其参考产品低15%至30%。仅在2020年,生物类似药就节省了79亿美元的成本,随着更多生物类似药进入市场,预计未来几年节省的成本将大幅增加。据 Cardinal Health 称,预计到 2025 年,生物仿制药将使美国药品支出减少 1330 亿美元。因此,在美国,生物仿制药具有巨大的潜力,可以降低生物药物的成本,使患者更容易获得护理,并创造新的创新和科学突破,从而推动该地区生物仿制药市场的增长。
关键参与者分析
生物仿制药市场分析包括安进公司、Celltrion 公司、赛诺菲公司、Biocon 有限公司、辉瑞公司、三星生物epis 有限公司、Coherus BioSciences 公司、礼来公司、山德士公司、梯瓦制药工业有限公司和雷迪博士实验室有限公司等参与者。在生物仿制药市场的参与者中,辉瑞公司和诺华公司凭借其提供的多元化产品组合位居前两位。
生物仿制药市场区域洞察
The Insight Partners 的分析师已详尽阐述了预测期内影响生物仿制药市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的生物仿制药市场细分和地域分布。
生物仿制药市场报告范围
| 报告属性 | 细节 |
|---|---|
| 市场规模 2021 | US$ 18.44 Billion |
| 市场规模 2028 | US$ 136.07 Billion |
| 全球复合年增长率 (2021 - 2028) | 34.8% |
| 历史数据 | 2019-2020 |
| 预测期 | 2022-2028 |
| 涵盖的领域 |
By 疾病适应症
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
生物仿制药市场参与者密度:了解其对业务动态的影响
生物仿制药市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求的驱动因素包括消费者偏好的演变、技术进步以及对产品优势的认知度的提升。随着需求的增长,企业正在扩展产品线,不断创新以满足消费者需求,并抓住新兴趋势,从而进一步推动市场增长。
- 获取 生物仿制药市场 主要参与者概述
生物仿制药市场中的公司广泛采用并购、产品上市等有机和无机策略。以下列出了一些近期的关键市场动态:
- 2022年1月,Biocon Ltd. 的子公司 Biocon Biologics 完成了对 Viatris 全球生物仿制药业务的收购。此次收购为 Biocon Biologics 提供了在发达市场和多个新兴市场的直接商业能力和支持基础设施,使其更贴近患者、客户和付款人。通过此次收购,Biocon Biologics 成为全球领先的生物仿制药公司,拥有八种已商业化的产品。
- 2022年10月,Biocon Biologics 将两项生物仿制药资产授权给 Yoshindo 在日本进行商业化。根据该交易条款,Yoshindo 获得了在日本对 Biocon Biologics 开发和生产的 bUstekinumab 和 bDenosumab 的独家商业化权利,潜在市场机会为 7 亿美元。
- 2022 年 12 月,Celltrion USA 宣布向 FDA 提交 CT-P13 新型皮下制剂的生物制品许可申请 (BLA)。皮下制剂具有通过提供高度一致的药物暴露和便捷的给药方法增强英夫利昔单抗药物治疗选择的潜力。
- 2022 年 9 月,Celltrion USA 获得美国 FDA 批准,其肿瘤生物仿制药 Vegzelma 用于治疗六种类型的癌症,例如转移性结直肠癌;复发性或转移性非鳞状非小细胞肺癌 (nsNSCLC);复发性胶质母细胞瘤;转移性肾细胞癌;持续性、复发性或转移性宫颈癌;以及上皮性卵巢癌、输卵管癌或原发性腹膜癌。Vegzelma 是 Celltrion 第三个获得美国 FDA 批准的肿瘤生物类似药。
- 2022 年 5 月,Biocon Biologics 和 Viatris 推出 Abevmy。Biocon Ltd. 的子公司 Biocon Biologics Ltd. 和 Viatris Inc. 宣布 Abevmy(bBevacizumab)已在加拿大上市。Abevmy 由 Biocon Biologics 和 Viatris 共同开发,是罗氏 Avastin(贝伐单抗)的生物类似药,已获得加拿大卫生部批准用于治疗四种肿瘤适应症。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
相关报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 生物仿制药市场
获取免费样品 - 生物仿制药市场