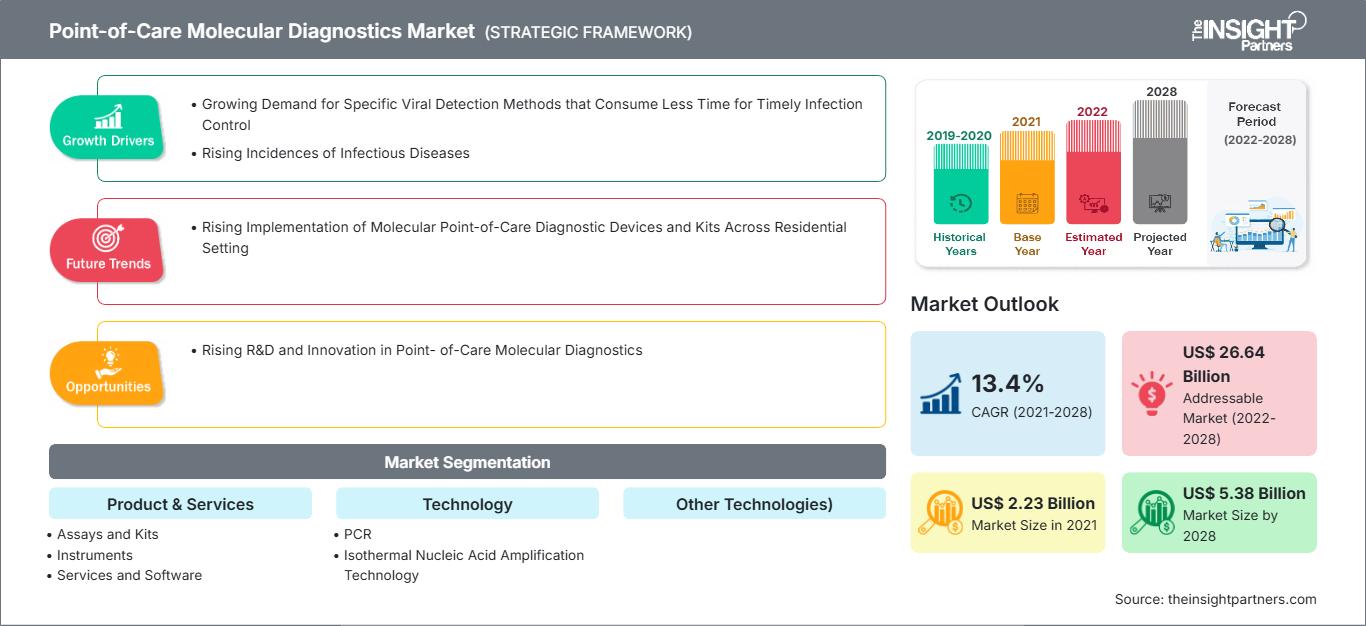
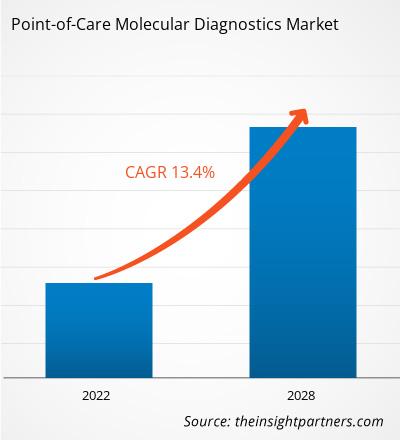
[研究报告]预计到 2028 年,即时诊断分子诊断市场规模将从 2021 年的 22.3094 亿美元增至 53.8118 亿美元;预计 2021 年至 2028 年的复合年增长率为 13.4%。
市场洞察和分析师观点:
即时诊断分子诊断包括便携式设备以及用于检测和诊断人体样本(例如咽拭子、血液、血清和粪便)中疾病的检测试剂盒。由于分子诊断简单、便捷、周转时间短以及改善患者预后的潜力,它正从集中式实验室转向分散式即时诊断分子检测。由于这些优势,它可以应用于资源匮乏或偏远地区的诊断。传染病发病率的上升极大地推动了对有效诊断的需求。在预测期内,对控制和消除传染病、及时发现病原体、实现有效治疗和疾病控制的诊断工具的需求不断增长,将推动市场发展。此外,现代技术正在使分子诊断领域发生显著变革。全球即时诊断分子诊断市场规模预计将从 2021 年的 22.3094 亿美元增至 2028 年的 53.8118 亿美元。预计 2021 年至 2028 年期间,该市场的复合年增长率将达到 13.4%。
增长动力与挑战:
传染病发病率上升
传染病的蔓延加快了检测的水平和速度。将传染病的分子诊断检测从实验室转移到即时诊断环境,有可能彻底改变检测的速度和数量。分子诊断是一种灵敏度更高的产品和服务,能够检测到较低浓度的传染性病原体,从而比以往更早地发现疾病。即时分子诊断有望最大程度地缩短获得可操作结果所需的时间,并促进早期感染检测、适当的感染控制措施以及参与治疗临床试验。甲型/乙型流感、呼吸道合胞病毒 (RSV) 和医院获得性感染 (HAI) 的流行率不断上升,推动了即时分子检测的需求。流感和呼吸道合胞病毒 (RSV) 即时检测可以改善患者治疗和感染控制。近期,即时分子诊断在 COVID-19 感染检测中发挥了至关重要的作用。对于 COVID-19 感染检测,基于 RT-PCR 的诊断测试耗时长、成本高昂,并且需要先进的设备和专业人员。高昂的诊断成本和检测试剂盒的匮乏使得监测社区传播变得困难。因此,迫切需要快速、经济且有效的方法来检测人群中的 COVID-19 病毒感染。简便有效的即时检测分子诊断设备可以进行现场检测,有助于预防感染并控制其传播。因此,对用于检测传染病的快速有效的即时检测分子试剂盒的需求不断增长,推动了市场的增长。
自定义此报告以满足您的要求
您将免费获得任何报告的定制,包括本报告的部分内容,或国家级分析、Excel 数据包,以及为初创企业和大学提供超值优惠和折扣
即时诊断分子诊断市场: 战略洞察
-
获取本报告的主要市场趋势。这个免费样本将包括数据分析,从市场趋势到估计和预测。
市场限制
报销削减带来的定价压力
目前,医疗诊断市场正面临各种诊断方法报销不足的问题,而这些方法对于患者的诊断至关重要。报销不足对市场产生了负面影响,主要经济体的大型市场增长停滞。全球各国的即时分子诊断测试都面临着类似的报销不足问题。报销体系通常对诊断测试不利。由于诊断测试报销不足,导致测试价格过低,最终降低了行业的盈利能力和市场规模。此外,低效的报销结构阻碍了更优质诊断测试的开发。此外,报销削减可能会对临床实践产生负面影响,因为临床实践既面临着不愿采用诊断方法的困境,也面临着未来缺乏改进测试的困境。此外,各国针对特定治疗都有各自的报销结构和政策。例如,美国和德国的医疗机构会向医院补偿治疗某种疾病的全部费用。因此,如果患者住院时间超过预期,医院将承担这笔费用。如果医院和医疗中心认为额外的检测会导致利润损失或每位患者的经济损失,这种情况可能会阻碍诊断工具的使用。因此,由于报销结构僵化,即时诊断分子诊断设备的原始设备制造商 (OEM) 面临着诸多官僚主义和定价挑战。因此,报销问题给即时诊断分子诊断设备制造商带来了定价压力,这成为即时诊断市场整体增长的主要制约因素。
报告细分和范围:
“全球即时诊断分子诊断”根据产品和市场细分。服务、技术、应用、最终用户和地域。基于产品和服务,全球即时分子诊断市场细分为检测和试剂盒、仪器以及服务和软件。基于技术的即时分子诊断市场细分为 PCR、等温核酸扩增技术 (INAAT) 和其他技术。基于应用的即时分子诊断市场细分为传染病、肿瘤学、血液学、产前检测、内分泌学和其他应用。基于最终用户的即时分子诊断市场细分为医院和诊所、诊断实验室、研究和学术机构等。
基于地域的健康市场中的即时分子诊断商业细分为北美(美国、加拿大和墨西哥)、欧洲(德国、法国、意大利、英国、俄罗斯和欧洲其他地区)、亚太地区(澳大利亚、中国、日本、印度、韩国和亚太其他地区)、中东和非洲(南非、沙特阿拉伯、阿联酋以及中东和非洲其他地区)和南美洲及中美洲(巴西、阿根廷以及南美洲和中美洲其他地区)
细分分析:
根据技术,分子诊断即时诊断市场细分为PCR、等温核酸扩增技术 (INAAT) 和其他技术。2021年,PCR技术可能占据最大的市场份额,但预计在预测期内,INAAT技术的增长速度最快。核酸等温扩增技术可在恒温条件下快速高效地积累核酸序列。该扩增技术是作为PCR的替代方案而开发的,用于生物传感靶标,例如DNA、RNA、小分子、蛋白质、细胞和离子。通过等温扩增方法产生的扩增子可用于构建多功能核酸纳米材料,该材料在生物医学、生物成像和生物传感领域具有广泛的应用。临床样本生化性质复杂,核酸靶点丰度低,且现有的生物传感器技术表明,需要某种形式的核酸扩增才能从用于即时检测的少量样本中获得临床相关的灵敏度。
基于产品和服务,即时分子诊断市场细分为检测试剂盒、仪器以及服务和软件。2021年,检测试剂盒细分市场可能占据最大的市场份额,预计在预测期内将以最快的速度增长。即时分子诊断检测试剂盒专为医生办公室、医院重症监护病房、门诊和社区卫生站设计。这些检测试剂盒有助于早期诊断呼吸道感染以及女性健康和性健康问题。检测试剂盒主要用于生命科学研究、环境监测以及药物研发。它们还用于各种应用,例如研究疾病途径、筛选潜在候选药物以及评估生物制药生产工艺。POC ELISA(酶联免疫吸附试验)试剂盒广泛用于检测和定量样品中的蛋白质和抗原。靶向特异性 ELISA 试剂盒用于简化免疫检测实验。
基于应用的即时诊断分子诊断市场细分为传染病、肿瘤学、血液学、产前检测、内分泌学和其他应用。2021 年,传染病领域可能占据最大的市场份额,然而,预计肿瘤学将在未来几年以最快的速度增长。全球约有 5000 万人患有癫痫,使其成为全球最常见的神经系统疾病之一。北美年龄调整后的癫痫发病率为十万人年 16 至 51 人。年龄调整患病率从千分之二点二到千分之四十一不等,具体取决于报告的国家。部分性癫痫可能占到新发癫痫的三分之二。社会经济地位较低的人群发病率较高。大约 25% 到 30% 的新发癫痫被认为是由其他原因引起的或继发于其他原因。癫痫发病率在年轻人和老年人群中最高,并且在 50 岁以后稳步上升。老年人癫痫发作和癫痫的最常见原因是脑血管疾病。
苯二氮卓类药物,例如地西泮、咪达唑仑或劳拉西泮,可作为治疗持续性癫痫的一线药物。即使在完成了备受期待的苯二氮卓类药物难治性癫痫持续状态随机试验——既定状态癫痫持续状态治疗试验 (ESETT) 之后,最佳二线药物仍不清楚。二线药物包括磷苯妥英、丙戊酸、左乙拉西坦等。
即时诊断分子诊断市场分析,区域分析:
根据地域划分,全球即时诊断分子诊断市场分为五个主要区域:北美、欧洲、亚太地区、南美和中美、中东和非洲。在北美,美国是即时诊断分子诊断市场的最大市场。可穿戴医疗设备、芯片实验室技术的引入以及智能手机使用量的增加推动了美国市场对即时诊断分子诊断设备的需求。在未来几年中,随着用于识别艾滋病毒、结核病和疟疾等传染病的快速检测日益普及,该行业将发生转变,使临床医生和患者能够在智能手机上查看结果并做出适当的治疗决策。诊断行业的最新进展正在推动美国即时诊断分子诊断市场的发展,旨在快速诊断,以便快速制定临床决策,从而辅助制定治疗方案。此外,美国是受新冠疫情影响最严重的国家之一。为了及时治疗,需要快速检测和发现病毒。全球各地,包括美国,都迫切需要能够实现分散、快速、灵敏且低成本的新冠肺炎感染诊断的即时诊断 (POC) 技术。因此,新冠疫情为美国即时诊断分子诊断市场带来了丰厚的增长机会。此外,美国慢性病发病率的快速上升也是推动市场发展的主要因素。例如,根据美国癌症协会的数据,2020 年美国估计有 180 万例新诊断癌症病例。乳腺癌、肺癌和支气管癌、前列腺癌、结肠癌和直肠癌、黑色素瘤以及肝癌是常见的癌症。
此外,根据美国疾病控制与预防中心 2020 年发布的研究,大约有 3420 万美国人患有糖尿病。根据同一项研究,美国青少年患糖尿病的几率更高。此类疾病的流行将推动该国对即时分子诊断的需求。此外,近年来,创新和增强的医疗技术显着增加。由于这种扩展,开发了先进的医疗设备,并刺激了医疗保健行业的发现和突破。此外,美国拥有多家致力于尖端即时分子诊断的公司。预计这一因素将进一步推动美国即时分子诊断市场的发展。
实时PCR (qPCR) 的引入扩展了分子诊断在医疗领域的应用范围。近年来,美国市场突破性的检测手段源于即时检测 (POC) 或患者附近进行的分子诊断检测。分子检测可以提高传统患者附近快速诊断检测的特异性和灵敏度,从而将美国即时分子诊断市场推向新的高度。此类诊断检测在实验室环境中的应用日益广泛,预计将增加其需求。对特定疾病的早期和精确诊断以提供适当治疗的需求不断增长,这将导致新技术的出现,从而推动美国 POC 分子诊断市场的发展。
行业发展和未来机遇:
全球即时诊断分子诊断市场主要参与者采取的各种举措如下:
- 2021 年 2 月,罗氏宣布向 FDA 申请 SARS-CoV-2 快速抗原检测的紧急使用授权,使医疗保健专业人员能够在即时诊断时快速做出决策。
- 2021 年 3 月,生物梅里埃宣布其专门从事分子综合征传染病检测的子公司 BioFire Diagnostics 已获得美国食品药品监督管理局 (FDA) 对 BIOFIRE® 的 De Novo 授权; RP2.1 检测组。
- 2020 年 11 月,Enzo Biochem 宣布了一项分析结果,该结果显示,在其专有的 GENFLEX™ 分子诊断平台上进行的测试能够成功检测出目前已知的 COVID-19 变体。虽然该公司的 PCR 检测无法区分不同的变体,但可以进一步分析阳性样本以识别变体。目前市场上的快速抗原检测不具备此功能。
- 2021 年 5 月,Biocartis Group NV 宣布与全球科学主导的生物制药公司阿斯利康 (LSE/STO/Nasdaq: AZN) 签署了一项新协议,旨在为 Biocartis 欧洲和全球分销商市场中的部分医院提供快速易用的 Idylla™ EGFR 检测产品,以支持识别 EGFR 突变患者。
即时诊断分子诊断市场区域洞察
The Insight Partners 的分析师已详尽阐述了预测期内影响即时诊断分子诊断市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的即时诊断分子诊断市场细分和地域分布。
即时诊断分子诊断市场报告范围
| 报告属性 | 细节 |
|---|---|
| 市场规模 2021 | US$ 2.23 Billion |
| 市场规模 2028 | US$ 5.38 Billion |
| 全球复合年增长率 (2021 - 2028) | 13.4% |
| 历史数据 | 2019-2020 |
| 预测期 | 2022-2028 |
| 涵盖的领域 |
By 产品与服务
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
即时诊断分子诊断市场参与者密度:了解其对业务动态的影响
即时诊断分子诊断市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求的驱动因素包括消费者偏好的演变、技术进步以及对产品优势的认知度的提升。随着需求的增长,企业正在扩展产品线,不断创新以满足消费者需求,并抓住新兴趋势,从而进一步推动市场增长。
- 获取 即时诊断分子诊断市场 主要参与者概述
美国是北美新冠病例最多的国家。这对该地区的各个行业以及供应链和分销链都产生了负面影响。疫情期间,生命科学公司将重点转向研发用于治疗危及生命疾病的新型药物。此外,对快速检测设备的需求也有所增加,这对北美即时分子诊断市场的增长起到了重要作用。此外,新冠疫情的持续蔓延也刺激了即时分子诊断试剂盒的需求。这些试剂盒的普及正在推动新产品的开发和上市。2021年3月,欧陆金融旗下的Clinical Enterprise, Inc.获得了美国食品药品监督管理局(FDA)颁发的紧急使用授权(EUA),用于其EmpowerDX COVID-19家用采集试剂盒的直接面向消费者(DTC)版本。同样,2020年7月,Eurofins USA 的临床诊断部门宣布推出用于检测 SARS-CoV-2 的混合 PCR 检测,这将大幅降低客户每次 PCR 检测的成本。
竞争格局和主要公司:
全球即时诊断分子诊断市场的一些知名企业包括 bioMérieux SA、F. Hoffmann-La Roche Ltd.、Danaher Corporation、Enzo Biochem, Inc.、Abbott、binx health, Inc.、Meridian BioScience, Inc.、Biocartis、Quidel Corporation 和 Bio-Rad Laboratories, Inc. 等。这些公司专注于新产品的推出和地域扩张,以满足全球不断增长的消费者需求,并扩大其专业产品组合的范围。它们拥有广泛的全球影响力,这使得它们能够服务于大量客户,从而提高其市场份额。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 即时诊断分子诊断市场
获取免费样品 - 即时诊断分子诊断市场