Regenerative Medicine Market Growth, Trends, Revenue & Forecast 2025-2031
Regenerative Medicine Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage:By Product Type [Cell-Based Products (Stem Cell and Cell-Based Immunotherapy), Gene Therapy, Tissue Engineering], Application (Oncology, Neurological Disorders, Wound Healing & Skin Regeneration, Ophthalmology, Orthopedics & Musculoskeletal, Immunology, Genetic Disorders, and Others), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Sep 2025
- Report Code : TIPRE00040655
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 237
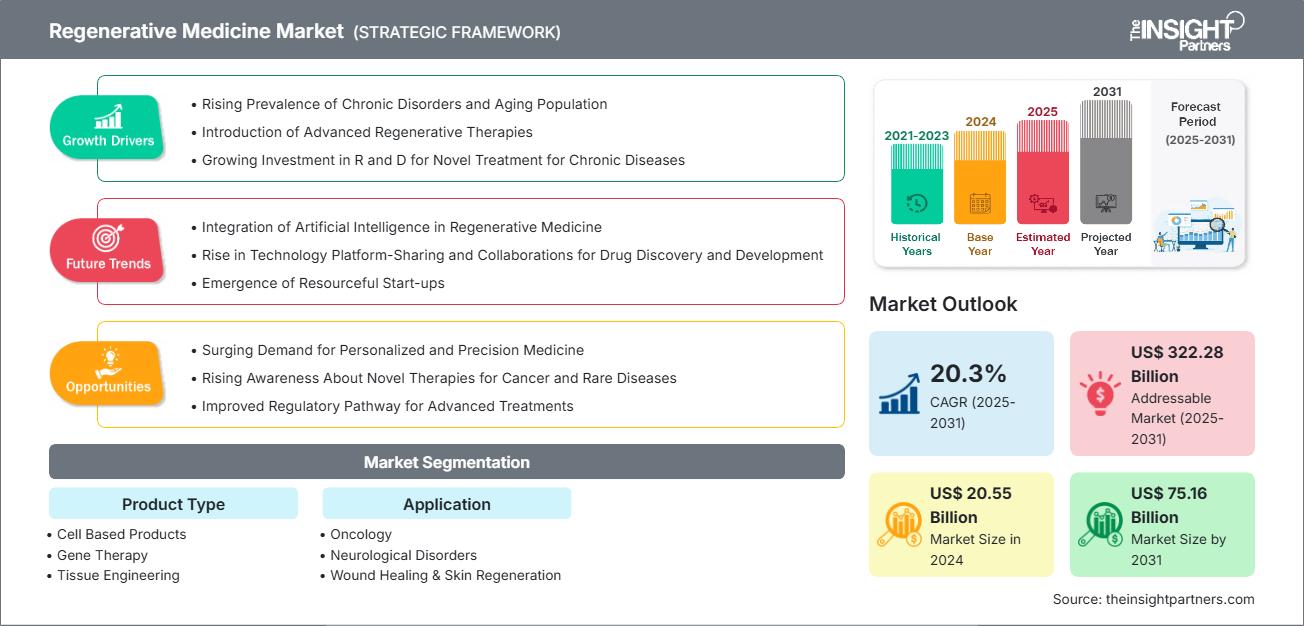
The Regenerative Medicine Market size is projected to reach US$ 75.16 billion by 2031 from US$ 20.55 billion in 2024. The market is expected to register a CAGR of 20.3% from 2025 to 2031.
Regenerative Medicine Market Analysis
The rising prevalence of chronic disorders, the surging aging population, and the introduction of advanced regenerative therapies are driving the regenerative medicine market growth. Additionally, increasing developments and adoption of stem cell and gene therapy contribute to market expansion. The surging demand for personalized and precision medicine is expected to create opportunities for the market in the coming years.
Regenerative Medicine Market Overview
North America is projected to dominate the regenerative medicine market, accounting for the largest share during the forecast period. Asia Pacific is expected to register a significant CAGR during the forecast period, owing to the government efforts boosting the adoption of regenerative medicine and an increase in research on stem cell therapy for various applications. China's aging population, along with the increasing prevalence of degenerative diseases, has intensified the demand for innovative regenerative therapies. These therapies repair or replace damaged tissues and organs. China has become a central hub for clinical trials, especially in cell and gene therapies. There are over 400 CAR trials for T-cell therapies targeting hematologic cancers and solid tumors, emphasizing the nation's critical role in advancing regenerative medicine.
AstraZeneca's US$ 2.5 billion investment in a research and development center in Beijing, announced in March 2025, and collaborations with the Korea Stem Cell Research Center, bolster China's research capabilities in this area. In November 2023, a clinical trial sponsored by Peking University Third Hospital in China investigated using dental pulp mesenchymal stem cells to treat chronic periodontitis patients. Researchers aimed to assess the safety of these administration protocols for the same patient population. An exploratory objective of this study was to investigate the effects of human dental pulp mesenchymal stem cells on biomarkers found in the gingival crevicular fluid of patients with chronic periodontitis. Innovations such as ActivSkin, bone repair scaffolds, and CAR-T therapies showcase China's capability to create and implement cutting-edge solutions. Introducing new products and ongoing clinical trials broadens treatment options for neurodegenerative diseases, cardiovascular disorders, and cancers.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONRegenerative Medicine Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Regenerative Medicine Market Drivers and Opportunities
Market Drivers:
- Rising Prevalence of Chronic Disorders and Aging Population The Cleveland Clinic reported nearly 1,000 isolated coronary artery bypass grafting (CABG) surgeries in 2024, with operative mortality rates lower than anticipated, highlighting the rising volume of surgeries and improved surgical outcomes. Regenerative therapies such as stem cell treatments and bioengineered tissues improve heart tissue repair post-infarction or after surgical procedures and reduce the need for repeat surgeries or lifelong medication.
- Introduction of Advanced Regenerative Therapies Regenerative medicine uses stem cells and technologies such as engineered biomaterials and gene editing to repair or replace damaged cells, tissues, or organs. Stem cell-based methods are being developed in laboratories worldwide, and some have already progressed to clinical trials. Introducing such regenerative therapies drives their adoption in clinical and medical settings as new disease treatments and drug development processes.
- Advancements in Stem Cell and Gene Therapy Advancements in stem cell and gene therapy enable targeted, personalized treatments for chronic and genetic diseases. Innovations such as iPSCs and CRISPR allow for tissue regeneration, disease correction at the genetic level, and reduced risk of rejection. These breakthroughs accelerate clinical applications and expand therapeutic possibilities across multiple medical fields.
Market Opportunities
- Surging Demand for Personalized and Precision Medicine Precision medicine tailors regenerative therapies to an individual's unique genetic, molecular, and cellular profile, which enhances treatment precision, safety, and effectiveness. The biomedical sector can advance targeted therapeutic interventions by integrating precision medicine with regenerative approaches. This fusion allows for the development of treatments that restore or substitute compromised tissues and organs and align with the unique physiological attributes of individual patients.
- Regulatory Support and Fast-Track Approvals Programs such as the FDA's RMAT designation and EMA's PRIME initiative accelerate the development and commercialization of innovative therapies. These pathways reduce time-to-market, lower development costs, and encourage investment, making it easier for companies to bring advanced regenerative treatments to patients with unmet medical needs.
- Strategic Collaborations and Licensing Partnerships between biotech firms, pharmaceutical companies, and research institutions enable shared resources, reduced R&D costs, and faster innovation. These alliances facilitate access to advanced technologies, expand therapeutic pipelines, and accelerate the commercialization of regenerative therapies across diverse medical applications and global markets.
Regenerative Medicine Market Report Segmentation Analysis
The regenerative medicine market is divided into different segments to give a clearer view of how it works, its growth potential, and the latest trends. Below is the standard segmentation approach used in industry reports:
By Product Type:
- Cell-Based Products Cell-based therapies use living cells to either treat or prevent diseases. They work by replacing damaged cells, restoring function, or modulating the body's cells. These therapies can involve cells, including stem and differentiated cells, and can be either autologous—using the patient's cells—or allogeneic—using cells from a donor.
- Gene Therapy Gene therapy products repair or replace damaged tissues and organs by delivering genetic material to a patient's cells. These therapies utilize viral vectors or other gene delivery systems to modify cells outside the body (ex vivo) or inside the body (in vivo). Viral vectors, particularly lentiviral and adenoviral vectors, are pivotal in gene delivery for therapies targeting genetic disorders and cancers.
- Tissue Engineering Tissue engineering products encompass scaffolds, bio-matrices, skin grafts, cartilage and bone regeneration products, and advanced 3D bioprinted tissues and organs. These repair, regenerate, or replace damaged tissues and organs by providing biocompatible frameworks and living cells that support natural healing and regeneration processes.
By Application:
- Oncology
- Neurological Disorders
- Wound Healing and Skin Regeneration
- Ophthalmology
- Orthopedics and Musculoskeletal
- Immunology
- Genetic Disorders
- Others
By Geography:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
The regenerative medicine market in North America is expected to hold a significant share of the market. The rising prevalence of chronic and degenerative conditions increases the demand for novel treatment approaches, driving market growth.
Regenerative Medicine
Regenerative Medicine Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 20.55 Billion |
| Market Size by 2031 | US$ 75.16 Billion |
| Global CAGR (2025 - 2031) | 20.3% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Regenerative Medicine Market Players Density: Understanding Its Impact on Business Dynamics
The Regenerative Medicine Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Regenerative Medicine Market Share Analysis by Geography
Asia Pacific is witnessing the fastest growth in the market. Emerging markets in Latin America, the Middle East, and Africa have many untapped opportunities for regenerative medicine providers to expand.
The regenerative medicine market growth differs in each region due to the increasing prevalence of chronic disorders, aging population, and introduction of advanced regenerative therapies. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds a significant portion of the global market
-
Key Drivers:
- Technological Advancements
- Supportive Regulatory Environment
- Adoption and Commercialization Infrastructure
- Trends: Shift Toward Personalized and Precision Medicine
2. Europe
- Market Share: Substantial share due to the Increasing prevalence of chronic and age-related diseases
-
Key Drivers:
- High Prevalence of Chronic and Age‑Related Diseases
- Favorable government policies and reimbursement frameworks
- Collaborative Research and Development Ecosystems
- Trends: Exosome-Based Therapeutics and Personalized Medicine
3. Asia Pacific
- Market Share: Fastest-growing region with a rising market share every year
-
Key Drivers:
- Rising Prevalence of Chronic Diseases
- Technological Advancements
- Government Support and Funding
- Trends: Advancements in Bioprinting and Tissue Engineering
4. South and Central America
- Market Share: Growing market with steady progress
-
Key Drivers:
- Advancements in Biotechnology and Research
- Investment in Healthcare Infrastructure
- Trends: Integration into Bioconvergence Ecosystem
5. Middle East and Africa
- Market Share: Although small, but growing quickly
-
Key Drivers:
- Increasing aging population
- Infrastructure Expansion and Technology Adoption
- Ambitious Government Initiatives & Investment in Healthcare
- Trends: Gene Editing and Cellular Engineering Expand Applications
Regenerative Medicine Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is strong due to the presence of established players such as Bristol-Myers Squibb Co; Novartis AG; Johnson & Johnson; Daiichi Sankyo Co Ltd; Takeda Pharmaceutical Co Ltd; Japan Tissue Engineering Co., Ltd; Bluebird Bio Inc; JCR Pharmaceuticals Co. Ltd; Vertex Pharmaceuticals Inc; Ferring Pharmaceuticals; CSL Behring LLC; and BioMarin Pharmaceutical Inc. Other players also add to the competitive landscape across different regions.
This high level of competition urges companies to stand out by offering:
- Advanced Products
- Value-added services such as customization and sustainable solutions
- Competitive pricing models
- Compliance with regulatory guidelines
Opportunities and Strategic Moves
- Moving beyond traditional uses (e.g., bone or skin regeneration) into emerging areas such as neurodegenerative diseases, diabetes, or organ regeneration. Exploring cosmetic and aesthetic applications, which face fewer regulatory hurdles.
- Forming alliances with established pharma companies to access distribution channels. Acquiring smaller innovative startups to accelerate pipeline growth. Collaborating with technology companies for the integration of digital health and diagnostics.
- Countries are developing expedited pathways for regenerative therapies, recognizing their potential to address unmet medical needs. Partnerships between regenerative startups and established pharmaceutical companies can leverage research and development, distribution, and commercialization strengths.
Major Companies operating in the Regenerative Medicine Market are:
- Bristol-Myers Squibb Co
- Novartis AG
- Johnson & Johnson
- Daiichi Sankyo Co Ltd
- Takeda Pharmaceutical Co Ltd
- Japan Tissue Engineering Co., Ltd.
- Bluebird Bio Inc
- JCR Pharmaceuticals Co. Ltd.
- Vertex Pharmaceuticals Inc
- Ferring Pharmaceuticals
- CSL Behring LLC
- BioMarin Pharmaceutical Inc.
Disclaimer: The companies listed above are not ranked in any particular order.
Other companies analyzed during the course of research:
- SanBio Company Limited
- Orchard Therapeutics
- Iovance Biotherapeutics
- Cell Trans
- Sarepta Therapeutics
- Spark Therapeutics
- Krystal Biotech
- Gamida Cell
- Janssen
- Aurion Biotech Japan, LLC
- Enzyvant Therapeutics
- Takeda Pharmaceutical Company
- Stratatech
- Kite Pharma
- Nipro Corporation
- JCR Pharmaceuticals Co., Ltd
- Terumo Corporation
- Celgene
- AnGes, Inc
- Shenzhen SiBiono GeneTech
- Fibrocell Technologies
- Holostem Terapie Avanzate S.R.L
- Stempeutics Research
- Organogenesis, Inc. & Novartis AG
- Sanofi (Genzyme Biosurgery)
- Cleveland Cord Blood Center
- Duke University School of Medicine
- New York Blood Center (NYBC)
- Neurotech Pharmaceuticals, Inc
Regenerative Medicine Market News and Recent Developments
- US Food and Drug Administration Approves Streamlined Patient Monitoring Requirements and Removal of REMS Programs within Bristol Myers Squibb's Cell Therapy Labels Bristol Myers Squibb announced that the US Food and Drug Administration (FDA) has approved label updates for both of its CAR T cell therapies, Breyanzi (lisocabtagene maraleucel; liso-cel) for the treatment of large B cell lymphoma (LBCL) and other lymphomas, and Abecma (idecabtagene vicleucel; ide-cel) for the treatment of multiple myeloma. These label updates reduce specific patient monitoring requirements and remove the Risk Evaluation and Mitigation Strategy (REMS) programs in place since each product was initially approved.
- Novartis to Acquire Anthos Therapeutics Novartis announced that it has reached an agreement to acquire Anthos Therapeutics, Inc., a clinical-stage biopharmaceutical company based in Boston. Anthos is developing abelacimab, a late-stage medication aimed at preventing stroke and systemic embolism in patients with atrial fibrillation. This transaction, subject to customary closing conditions, aligns with Novartis' growth strategy and focus on therapeutic areas, leveraging the company's expertise in cardiovascular health.
- bluebird bio Presents Positive Long-Term Data On LYFGENIA (lovotobegligene autotemcel) Gene Therapy for Sickle Cell Disease bluebird bio, Inc. announced new and updated data from LYFGENIA (lovotobegligene autotemcel, or lovo-cel) gene therapy for patients with sickle cell disease who have a history of vaso-occlusive events (VOEs). As of July 2024, 70 patients were treated across the complete lovo-cel clinical development program, with follow-up beyond 9 years in the earliest treated patients.
Regenerative Medicine Market Report Coverage and Deliverables
The "Regenerative Medicine Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Regenerative Medicine Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Regenerative Medicine Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Regenerative Medicine Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the Regenerative Medicine Market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For