Blood Brain Barrier Technologies Market Outlook and Growth Forecast (2025-2034)
Blood Brain Barrier Technologies Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Bispecific Antibody RMT Approach, Trojan Horse Approach, Increasing Permeability, Passive Diffusion, and Other Non-Invasive BBB Technologies); Application (Alzheimer's Disease, Epilepsy, Parkinson's Disease, Multiple Sclerosis, Hunter's Syndrome, Brain Cancer, and Others), and Geography
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Mar 2026
- Report Code : TIPRE00021883
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
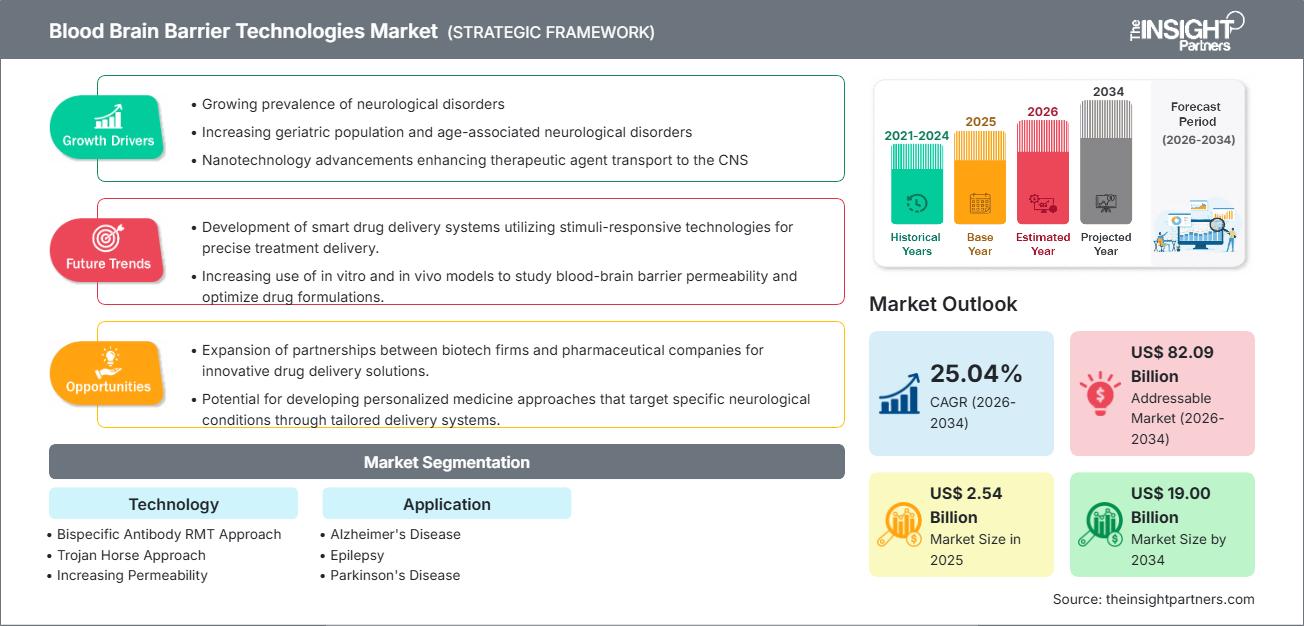
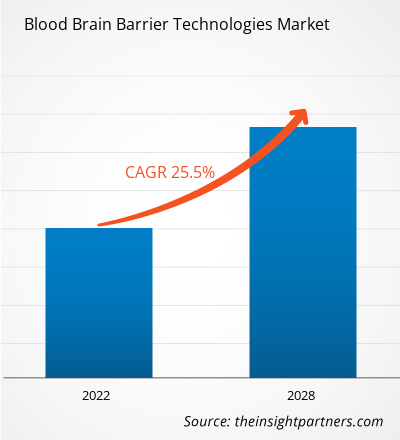
The Blood Brain Barrier (BBB) Technologies Market size is expected to reach US$19.00 billion by 2034 from US$2.54 billion in 2025. The market is anticipated to register an exceptional Compound Annual Growth Rate (CAGR) of 25.04% during the forecast period of 2026–2034.
Blood Brain Barrier Technologies Market Analysis
The Blood Brain Barrier technologies market forecast indicates explosive growth, driven by the increasing global prevalence of debilitating neurological disorders and the persistent challenge of delivering effective therapeutics to the Central Nervous System (CNS). The market expansion is primarily fueled by massive, strategic investments in neuroscience R&D by major pharmaceutical and biotechnology companies. These firms are rapidly adopting innovative drug delivery platforms, such as receptor-mediated transcytosis (RMT) and targeted nanoparticles, to overcome the BBB's natural protective mechanisms. This trend is further facilitated by regulatory agencies offering fast-track designations for novel CNS disease treatments, accelerating the development and commercialization of new technologies designed for targeted drug penetration.
Blood Brain Barrier Technologies Market Overview
The Blood Brain Barrier Technologies encompass a sophisticated array of methods and systems specifically designed to temporarily modulate or bypass the highly selective endothelial barrier that restricts drug access to the brain and spinal cord. This market includes biological approaches, such as Bispecific Antibodies and Trojan Horse vectors, which utilize endogenous transport pathways, as well as physical methods, such as Focused Ultrasound (FUS) and osmotic disruption, to enhance permeability. These technologies are crucial for unlocking the therapeutic potential of large molecule biologics (such as antibodies and peptides) and other agents that are typically ineffective against CNS diseases. By enabling precise and increased drug concentration within the brain parenchyma, technologies are set to revolutionize the treatment paradigms for chronic neurological conditions.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONBlood Brain Barrier Technologies Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Blood Brain Barrier Technologies Market Drivers and Opportunities
Market Drivers:
- Rising Prevalence of Neurological Disorders and High Unmet Need: The increasing global incidence of neurodegenerative diseases, including Alzheimer's, Parkinson's, and Multiple Sclerosis, creates an urgent, high-value demand for innovative delivery systems capable of treating these conditions, which have historically been undertreated due to the BBB challenge.
- Increasing Demand for Targeted Drug Delivery to the CNS: Modern pharmaceutical R&D is focused on high-specificity, low-toxicity therapeutics. BBB technologies enable the targeted delivery of these agents, minimizing systemic exposure and potential side effects while maximizing therapeutic concentrations at the disease site.
- Growing Investments in Biotechnology and Pharmaceutical R&D: There is a pronounced shift of R&D capital into neuroscience. Governments and private equity are heavily funding specialized biotech firms focused exclusively on developing proprietary BBB platforms, which de-risk CNS drug pipelines for larger pharmaceutical partners.
Market Opportunities:
- Development of Non-Invasive BBB Technologies for Improved Patient Compliance: Technologies such as Focused Ultrasound (FUS), which can temporarily and precisely open the BBB non-invasively, represent a significant opportunity. FUS promises to improve patient compliance and enable repeat dosing, especially compared to surgical or intraventricular delivery methods.
- Expansion in Emerging Markets with Rising Healthcare Infrastructure: As economies in the Asia-Pacific and Latin American regions mature, investments in specialized healthcare and access to advanced biologics are increasing. This opens new markets for BBB platforms, particularly in large, underserved patient populations.
- Strategic Partnerships for Accelerating Clinical Trials and Commercialization: Collaboration between biotech firms (possessing novel BBB platforms) and multinational pharmaceutical companies (with extensive clinical pipelines and commercialization scale) is crucial. Licensing agreements and joint ventures will accelerate the testing and market entry of BBB-enabled drugs.
Blood Brain Barrier Technologies Market Report Segmentation Analysis
The Blood Brain Barrier Technologies Market is segmented based on the core mechanism used to facilitate drug transport and the specific disease areas being targeted.
By Technology:
- Bispecific Antibody Receptor-Mediated Transcytosis (RMT) Approach
- Trojan Horse Approach
- Increasing Permeability
- Passive Diffusion
- Other Non-Invasive BBB Technologies
By Application:
- Alzheimer’s Disease (AD)
By Geography:
- North America
- Europe
- Asia Pacific
- South & Central America
- Middle East & Africa
Blood Brain Barrier Technologies Market Regional Insights
The regional trends and factors influencing the Blood Brain Barrier Technologies Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Blood Brain Barrier Technologies Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Blood Brain Barrier Technologies Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 2.54 Billion |
| Market Size by 2034 | US$ 19.00 Billion |
| Global CAGR (2026 - 2034) | 25.04% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Technology
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Blood Brain Barrier Technologies Market Players Density: Understanding Its Impact on Business Dynamics
The Blood Brain Barrier Technologies Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Blood Brain Barrier Technologies Market top key players overview
Blood Brain Barrier Technologies Market Share Analysis by Geography
North America holds the largest market share, while Asia-Pacific is poised to witness the fastest growth rate during the forecast period. The global dominance is largely shaped by regional R&D concentration, intellectual property rights, and regulatory environments favorable to high-risk, high-reward CNS therapies. Below is a summary of market share and trends by region:
North America
- Market Share: Dominates the Blood Brain Barrier Technologies Market, holding the largest share due to the highest concentration of leading pharmaceutical companies, vast R&D infrastructure, and significant venture capital funding dedicated to biotech startups specializing in BBB platforms.
- Key Drivers: Extensive pipeline development for Alzheimer's and Parkinson's disease.
- Trends: Strong emphasis on the clinical translation of advanced RMT and bispecific antibody technologies into late-stage trials.
Europe
- Market Share: Commands a significant share, driven by strong government-backed research initiatives (e.g., Horizon Europe funding for neuroscience) and a growing CNS disease burden across the region.
- Key Drivers: Strong academic collaboration between institutions and industry partners.
- Trends: Increasing adoption of non-invasive physical BBB modulation techniques, particularly focused ultrasound, being tested in European research hospitals.
Asia-Pacific
- Market Share: Fastest-growing region, owing to rapidly increasing investments in healthcare research, an expanding patient population for neurological disorders, and the establishment of dedicated biotechnology hubs.
- Key Drivers: Rapid growth in private biotechnology companies focusing on early-stage BBB innovation.
- Trends: High adoption of partnership models where local biotech firms collaborate with Western pharma companies to co-develop BBB drugs tailored for regional markets.
South and Central America
- Market Share: Emerging market with nascent but growing opportunities, primarily driven by the expansion of the pharmaceutical sector and improving access to specialized care.
- Key Drivers: Public-private health partnerships aimed at addressing neurological health disparities.
- Trends: Initial adoption of cost-effective Trojan Horse approaches and generic drug repurposing utilizing passive diffusion enhancers.
Middle East and Africa
- Market Share: Developing market with high growth potential, supported by substantial government investment in healthcare infrastructure expansion and a focus on establishing medical R&D centers.
- Key Drivers: National e-health strategies prioritizing specialized care, including neurology.
- Trends: Implementation of specialized neuroscience research programs that require access to cutting-edge BBB technologies, primarily through collaborations with global leaders.
Blood Brain Barrier Technologies Market Players Density: Understanding Its Impact on Business Dynamics
The competitive environment in the Blood Brain Barrier Technologies Market is highly dynamic, characterized by a mix of large pharmaceutical companies and small, specialized biotech platform developers. Competition is centered not only on commercializing the final drug but, crucially, on demonstrating the superior safety, efficacy, and scalability of the proprietary drug delivery technology itself.
This competitive environment pushes vendors to differentiate through:
- Validated Delivery Platforms: Demonstrating clinical safety and efficacy of the BBB technology in early-stage trials (Phase I and II) is the primary competitive advantage.
- Strategic Licensing and Partnerships: Smaller biotechs focus on licensing their proprietary BBB technology to Big Pharma to fund development and ensure broad market reach.
- Targeting Specific Receptors: Developing next-generation Bispecific Antibodies that minimize off-target effects and maximize brain uptake via novel or underutilized BBB receptors.
- Non-Invasive Modality Integration: Focusing on integrating physical disruption technologies (like FUS) with specific pharmaceutical candidates to offer a complete, non-surgical treatment solution.
Major Companies operating in the Blood Brain Barrier Technologies Market are:
- Teva Pharmaceutical Industries Ltd.
- F. Hoffmann-La Roche Ltd.
- Eli Lilly and Company
- Pfizer, Inc.
- Johnson and Johnson Services, Inc.
- Bristol-Myers Squibb Company
- Bioasis Technologies Inc.
- Fabre-Kramer Pharmaceuticals, Inc.
- Abliva AB
Disclaimer: The companies listed above are not ranked in any particular order.
Blood Brain Barrier Technologies Market News and Recent Developments
- F. Hoffmann-La Roche Ltd.: Roche has developed its proprietary Brainshuttle™ technology, which allows large therapeutic molecules such as antibodies and oligonucleotides to cross the blood–brain barrier. This platform is currently under clinical evaluation for neurodegenerative and rare CNS disorders, reinforcing Roche's leadership in targeted brain delivery solutions.
-
Pfizer, Inc.: Pfizer is developing innovative approaches to overcome the blood–brain barrier, such as AAV vectors and nanoparticle-based delivery systems. These technologies are targeted at the improvement of treatment options for brain metastases and rare neurological diseases, in tune with Pfizer’s strategic focus on gene therapy and oncology.
- Johnson & Johnson Services, Inc. : Johnson & Johnson highlighted its collaboration with VECT-HORUS, a platform that enables drug delivery across the blood–brain barrier. The technology is also under investigation for the treatment and diagnosis of various neurodegenerative disorders, furthering J&J’s commitment to CNS therapeutics.
Blood Brain Barrier Technologies Market Report Coverage and Deliverables
The "Blood Brain Barrier Technologies Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering below areas:
- Blood Brain Barrier Technologies Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope.
- Blood Brain Barrier Technologies Market trends, as well as market dynamics such as drivers, restraints, and key opportunities.
- Detailed PEST and SWOT analysis.
- Blood Brain Barrier Technologies Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments.
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the Blood Brain Barrier Technologies Market.
- Detailed company profiles.
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For