Pharma ADMET Testing Market Outlook & Key Trends 2034
Pharma ADMET Testing Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Testing Type (In Vitro ADMET Testing, In Silico ADMET Testing, In Vivo ADMET Testing); Technology (Cell Culture, High Throughput, OMICS Technology, Molecular Imaging); Application (Systemic Toxicity, Hepatotoxicity, Renal Toxicity, Neurotoxicity); and Geography
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Mar 2026
- Report Code : TIPRE00029509
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
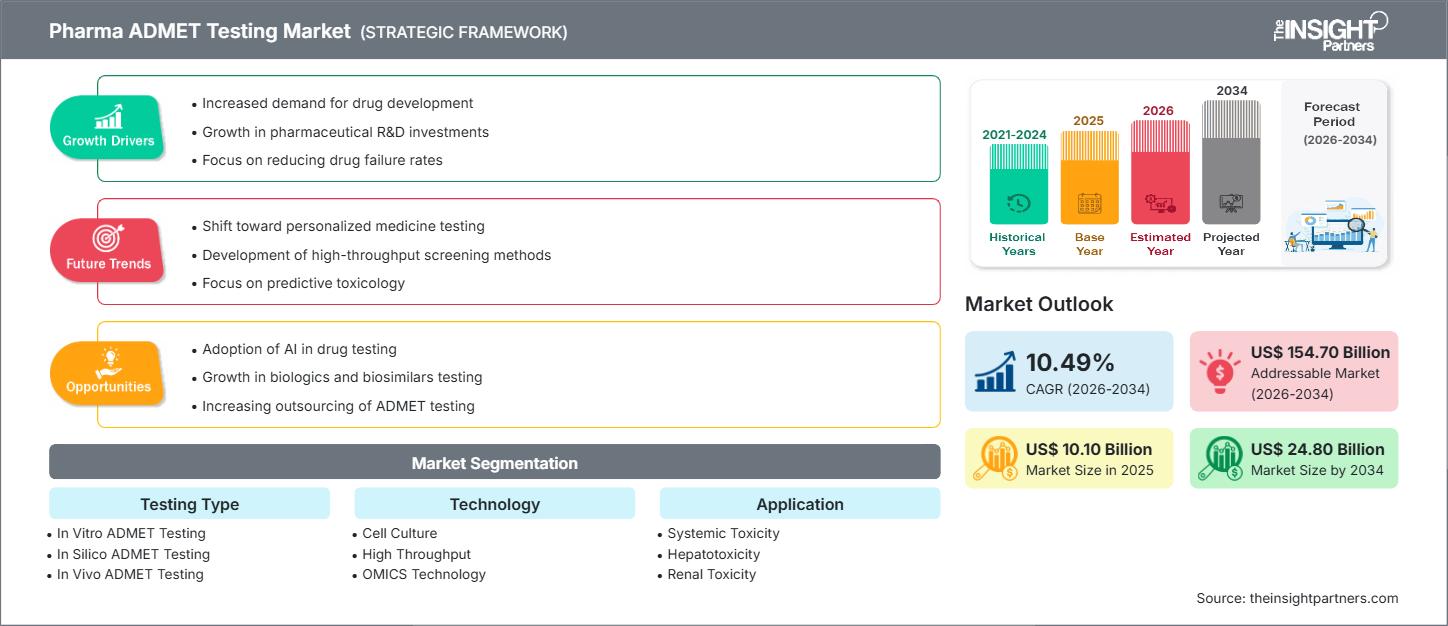
The pharma ADMET testing market size is expected to reach US$ 24.80 billion by 2034 from US$ 10.10 billion in 2025. The market is anticipated to register a CAGR of 10.49% during 2026–2034.
Pharma ADMET Testing Market Analysis
The Pharma ADMET Testing Market (Absorption, Distribution, Metabolism, Excretion, and Toxicity) is expanding quickly, primarily driven by the alarmingly high rate of late-stage drug failure and the increasing need to integrate safety and efficacy assessments early in the drug discovery process. Regulatory bodies, such as the FDA and EMA, are also mandating comprehensive ADMET screening. Technologies like in vitro models and In-silico/AI modeling are rapidly gaining traction to provide more physiologically relevant and cost-effective predictions than traditional in vivo (animal) testing. Outsourcing of these specialized studies to Contract Research Organizations (CROs) is a significant trend. The market is expected to grow rapidly with the advancement of high-throughput screening, the rising adoption of AI, and the global focus on precision medicine.
Pharma ADMET Testing Market Overview
Pharma ADMET Testing is a critical discipline in pharmacology and drug discovery that assesses how a drug candidate behaves in the body, determining its likelihood of success and safety profile. By filtering out compounds with poor pharmacokinetic or safety profiles early on, ADMET testing significantly reduces the time and multi-billion-dollar costs associated with late-stage clinical failures. Key methodologies include In Vivo (animal) studies, In Vitro, and In Silico. The shift towards In Vitro and In Silico models is being accelerated by ethical concerns over animal testing and advancements in predictive accuracy, such as the use of Organ-on-a-Chip technologies that better mimic human physiology.
Customizee This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONPharma ADMET Testing Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Pharma ADMET Testing Market Drivers and Opportunities
Market Drivers:
- High Rate of Late-Stage Drug Failure & Attrition: Approximately 95% of drug candidates fail clinical trials, with a substantial portion failing due to toxicity or poor ADME properties. This immense cost pressure drives the demand for early, highly predictive ADMET testing to de-risk development.
- Increasing Stringency of Regulatory Requirements: Regulatory bodies like the FDA and EMA are mandating thorough ADMET profiling, and recent updates (e.g., FDA Modernization Act 2.0 and ICH M12 guideline) are encouraging the inclusion of advanced in vitro and in silico evidence in drug submissions.
- Technological Advancements in Predictive Models: Innovations like 3D cell cultures, microfluidic-based Organ-on-a-Chip systems, and High-Throughput Screening (HTS) offer more human-relevant, efficient, and scalable testing, reducing reliance on expensive and less predictive animal models.
Market Opportunities:
- Integration of AI and Machine Learning: AI/ML platforms are transforming the market by accelerating lead optimization, predicting ADMET properties from chemical structure alone, and mining large datasets for real-time toxicity insights. This offers faster, cost-effective, and highly predictive screening.
- Growing Focus on Personalized Medicine: The demand for personalized treatments requires ADMET testing to assess individual drug responses and metabolism variability (Pharmacogenomics). This fuels the need for more precise and patient-specific testing methods.
- Expansion of Contract Research Organizations (CROs): Pharmaceutical and biotechnology companies are increasingly outsourcing ADMET studies to CROs to leverage specialized expertise, advanced technology infrastructure, and cost efficiencies, driving growth in the services segment.
Pharma ADMET Testing Market Report Segmentation Analysis
The Pharma ADMET Testing Market is typically segmented as follows:
By Testing Type:
- In Vitro ADMET Testing: Uses non-living systems, such as cell cultures, tissue samples, and biochemical assays. This is the largest segment and is growing due to ethical and regulatory support.
- In Silico ADMET Testing: Utilizes computational models, Quantitative Structure-Activity Relationship (QSAR) models, and AI/Machine Learning algorithms to predict properties based on chemical structure.
- In Vivo ADMET Testing: Involves the use of living organisms to study whole-body effects, primarily used for final safety confirmation.
By Technology:
- Cell Culture: Includes traditional 2D cell lines, 3D spheroids, and Organoids; offers a more physiologically relevant environment.
- High Throughput: Automated systems for rapidly testing thousands of compounds simultaneously for specific ADMET endpoints.
- OMICS Technology: Uses genomics, proteomics, and metabolomics for detailed molecular toxicology studies and biomarker identification.
- Molecular Imaging: Techniques used to visualize and measure drug distribution and metabolism in real-time.
By Application:
- Systemic Toxicity: Assessment of toxic effects on the entire body or multiple organ systems (often the largest segment).
- Hepatotoxicity: Testing for drug-induced liver injury, a leading cause of drug attrition.
- Renal Toxicity: Testing for drug-induced kidney injury.
- Neurotoxicity: Testing for adverse effects on the nervous system.
By Geography:
- North America
- Europe
- Asia-Pacific
- South & Central America
- Middle East & Africa
The regional trends and factors influencing the Pharma ADMET Testing Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Pharma ADMET Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Pharma ADMET Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 10.10 Billion |
| Market Size by 2034 | US$ 24.80 Billion |
| Global CAGR (2026 - 2034) | 10.49% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Testing Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Pharma ADMET Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Pharma ADMET Testing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Pharma ADMET Testing Market top key players overview
Pharma ADMET Testing Market Share Analysis by Geography
North America is anticipated to dominate the Pharma ADMET Testing Market. The dominance of this region is driven by the presence of a robust pharmaceutical and biotechnology industry, substantial R&D investments, a high concentration of leading CROs, and the early adoption of advanced technologies like AI and Organ-on-Chip platforms, often supported by stringent FDA regulations.
The Asia-Pacific (APAC) region is expected to register the highest growth during the forecast period. This rapid growth is fueled by increasing investments in pharmaceutical R&D, rising outsourcing of drug development activities, government initiatives supporting biotech innovation, and an improving regulatory landscape.
Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds the largest market share, driven by robust IT infrastructure and a concentration of global pharma/biotech companies.
- Key Drivers: High R&D spending, early adoption of AI/HTS screening, and strong regulatory standards (FDA).
- Trends: Rapid integration of AI for predictive toxicology and the adoption of Organ-on-Chip technologies.
2. Europe
- Market Share: Significant market share, driven by strong regulatory compliance requirements and public push for non-animal testing.
- Key Drivers: Stringent data privacy and animal welfare regulations (e.g., EU push for non-animal testing), and strong academic-industry collaboration.
- Trends: Focus on validated in vitro models (NAMs), transparent testing data, and specialized ADMET services for biologics.
3. Asia Pacific
- Market Share: The fastest-growing regional market, fueled by pharmaceutical outsourcing and local drug development.
- Key Drivers: Government-backed R&D investment (especially in China, India, and South Korea), lower cost of services, and increasing prevalence of chronic diseases.
- Trends: Expansion of CRO capabilities, growth in generics and biosimilars testing, and increased use of in silico platforms.
4. South and Central America
- Market Share: Emerging region with growing adoption of outsourced R&D.
- Key Drivers: growing adoption of outsourced R&D.Increased digital marketing adoption across e-commerce and entertainment sectors.
- Trends: Expansion of affordable cloud-based AI solutions by global tech providers.
5. Middle East and Africa
- Market Share: Emerging market with strong potential, led by strategic national healthcare and digital transformation initiatives.
- Key Drivers: Major national digital and AI strategies fostering innovation in social engagement.
- Trends: AI-based audience sentiment tracking, influencer fraud detection, and multilingual content moderation through machine learning.
Pharma ADMET Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Pharma ADMET Testing Market is highly competitive, featuring a mix of large, diversified life science and technology companies, specialized Contract Research Organizations (CROs), and innovative technology/software start-ups. Major players are focused on integrating cutting-edge technologies and offering comprehensive end-to-end services.
The competitive landscape is driving vendors to differentiate through:
- Vendors are heavily investing in computational tools to provide high-accuracy in silico prediction of toxicity and ADME properties, dramatically speeding up lead optimization.
- Differentiation through advanced systems like 3D cell cultures, organoids, and microfluidic Organ-on-Chip (OOC) devices that provide a more human-relevant testing environment.
- CROs are expanding their portfolios to offer seamless services from early-stage ADMET screening through to clinical trials, acting as comprehensive partners for pharmaceutical companies.
Opportunities and Strategic Moves
- Strategic Acquisitions and Partnerships: Large players and CROs are actively acquiring or partnering with AI/ML and OOC technology start-ups to quickly integrate innovative, next-generation ADMET testing capabilities into their service offerings.
- Focus on Biologics and Novel Modalities: Companies are developing specialized ADMET testing platforms tailored for complex large molecules like Biologics, Cell and Gene Therapies, and Antibody-Drug Conjugates (ADCs).
- Geographic Expansion: Vendors are expanding their testing facilities and service offerings in high-growth regions like Asia-Pacific to capitalize on increasing outsourcing and local R&D activity.
Major Companies Operating in the Pharma ADMET Testing Market Are:
- CMIC HOLDINGS Co., LTD
- Charles River Laboratories
- Wuxi AppTec
- Promega Corporation
- MERCK KGaA
- Agilent Technologies, Inc.
- Biovia (Dassault Systèmes)
- Cyprotex Limited
- Bio-Rad Laboratories, Inc.
Disclaimer: The companies listed above are not ranked in any particular order.
Pharma ADMET Testing Market News and Recent Developments
- For instance, in May 2023, WuXi AppTec Drug Metabolism and Pharmacokinetics (DMPK) officially opened a brand-new R&D center in Nantong, China. This R&D center will focus on conducting large animal PK studies as well as non-GLP bioanalytical research services. The launch of the DMPK Nantong R&D Center has expanded the capabilities and capacity of WuXi AppTec’s DMPK Services Department. This will allow the DMPK Department to continue to provide comprehensive and high-standard PK study services and accelerate the new drug development process for clients.
Pharma ADMET Testing Market Report Coverage and Deliverables
The "Pharma ADMET Testing Market Size and Forecast (2024–2031)" report provides a detailed analysis of the market covering below areas:
The "Pharma ADMET Testing Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering below areas:
- Pharma ADMET Testing Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Pharma ADMET Testing Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Pharma ADMET Testing Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the Pharma ADMET Testing Market. Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For