The North America acute lung injury market is expected to grow from US$ 201.93 million in 2021 to US$ 280.16 million by 2028; it is estimated to grow at a CAGR of 4.8% from 2021 to 2028.
The US, Canada, and Mexico are economies in North America. Growing geriatric population is expected to surge the market growth. The degeneration of various cells or tissues with age leads to a greater risk of multiple diseases. Elderly people are more prone to get affected by the failure of organs such as the lungs, heart, and kidney. This is mainly ascribed to the changes in the composition and metabolic activities in the ecosystem of the aging human body. With the increasing age, the incidence of organ systems efficiency decreases resulting prone to diseases such as respiratory diseases and other infection, thereby driving the target market. Thus, ALI is correlated with increased morbidity and mortality in the aging population. Also, age is generally reported as a risk factor for the growth of ARDS. Countries such as the US and Canada are experiencing significant growth in the geriatric population. The population is aging; over 65s are expected to account for 1 in 6 of the population by 2050, increasing 1 in 11 in 2019. With a strong association between aging and increased incidence of diseases—including respiratory conditions such as chronic obstructive pulmonary disease and acute infection—thorough research on the impact of disease risk and severity will be necessary to manage the health of an ever-aging population effectively. The growth of the geriatric population in these countries is driven by improved healthcare facilities and better healthcare services, which has resulted in increased life expectancy. Thus, the growing aging population is among the major factors for the growth of the North America acute lung injury market.In case of COVID-19, North America is highly affected especially the US. The high number of COVID cases have resulted in a negative impact on country’s and region’s economy and there has been a decline in overall business activities and growth of various industries operating in the region. Market players are initiated clinical trials for the drug development of acute lung injury. For instance, Chimerix, a biopharmaceutical company initiated a Phase II/III clinical trial in June 2020 for to evaluate the safety and efficacy of Dociparstat sodium (DSTAT) in patients with Acute Lung Injury (ALI) due to COVID-19. DSTAT is a glycosaminoglycan derivative of heparin with robust anti-inflammatory properties. Chimerix completes enrolment in its phase II part of phase II/III trial for acute lung injury. The Phase 2 portion of the study will enroll 24 subjects to confirm the maximum safe dose and then expand it to 50 patients at the selected dose. Contingent upon positive results, the Phase 3 portion of the study will enroll approximately 450 subjects. Furthermore, Altasciences is pleased to support ReAlta Life Sciences by conducting a Phase I trial to evaluate RLS-0071 for treatment of Acute Lung Injury (ALI) as a result of viral infections, such as COVID-19, respiratory syncytial virus (RSV), and influenza. As part of the ReAlta ALI program underway, the Phase I trial is a single-ascending dose, randomized, double-blind, placebo-controlled, adaptive-design study to evaluate the safety, tolerability, PK, and PD of RLS-0071 in healthy subjects, performed at the Altasciences clinical pharmacology unit in Montreal, Canada.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the North America acute lung injury market. The North America acute lung injury market is expected to grow at a good CAGR during the forecast period.
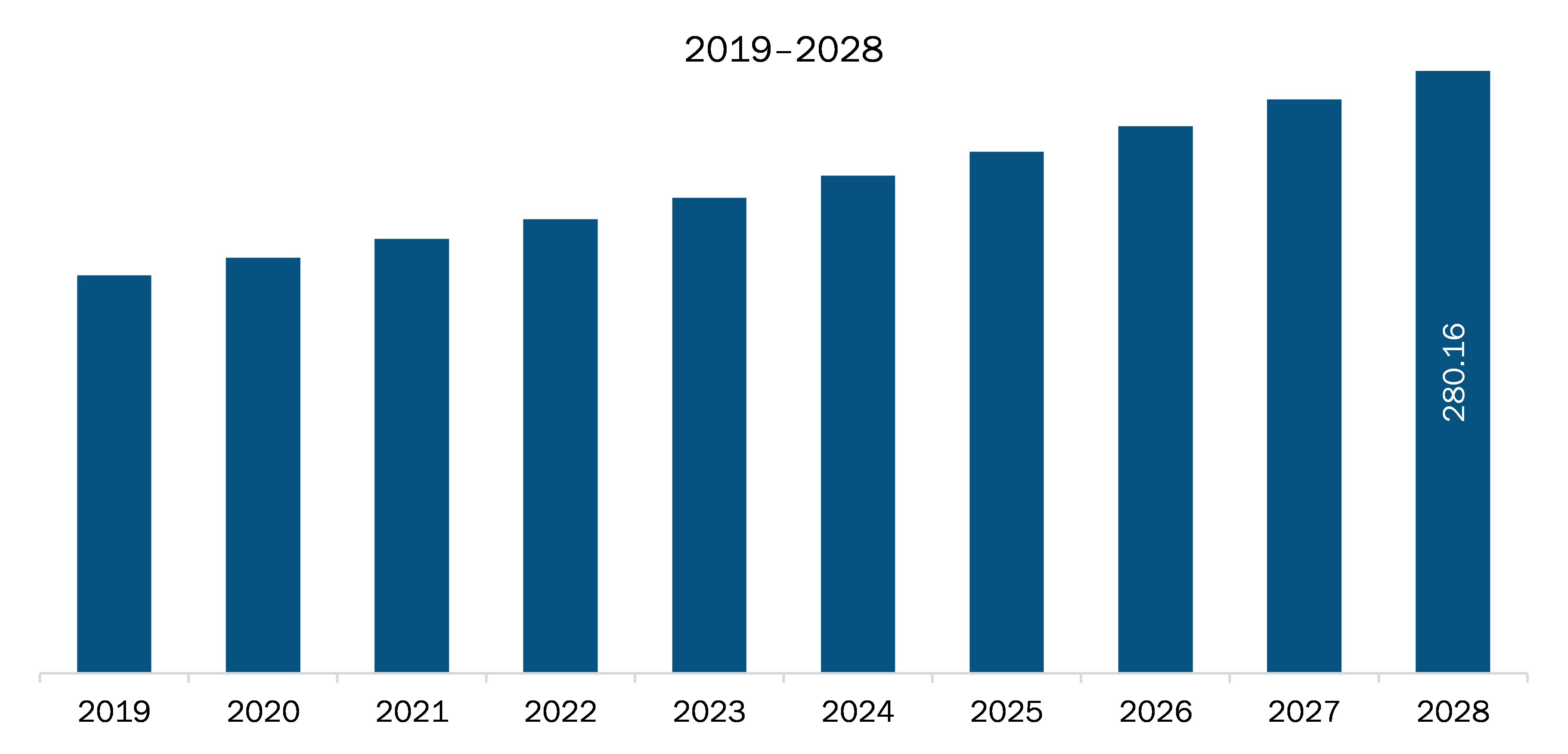
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
North America Acute Lung Injury Market Segmentation
North America Acute Lung Injury Market – By Therapy
- Mechanical Ventilation
- Fluid Management
- Pharmacotherapy
- Adjunctive Procedures
North America Acute Lung Injury Market – By End User
- Hospitals
- Ambulatory Surgery Centers
- Others
North America Acute Lung Injury Market, by Country
- US
- Canada
- Mexico
North America Acute Lung Injury Market - Companies Mentioned
- Angion
- Asklepion Pharmaceuticals, LLC.
- GlaxoSmithKline Plc
- Qx Therapeutics, Inc.
- ReAlta Life Sciences, Inc.
- Stemedica Cell Technologies, Inc
- Windtree Therapeutics, Inc.
North America Acute Lung Injury Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 201.93 Million |
| Market Size by 2028 | US$ 280.16 Million |
| CAGR (2021 - 2028) | 4.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Therapy
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For