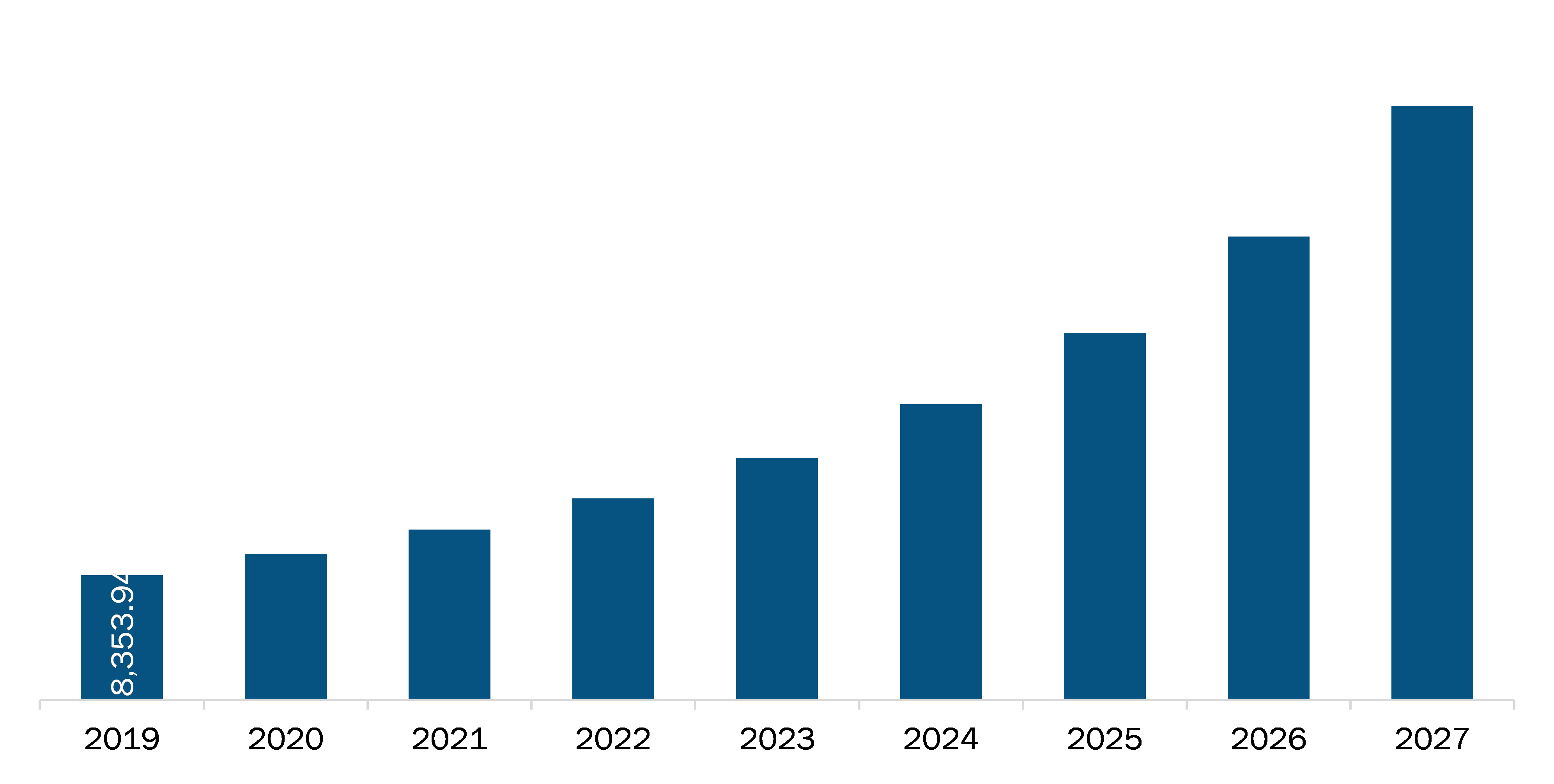
The North America Software as Medical Device market is expected to reach US$ 39,854.20 Mn in 2027 from US$ 8,353.94 Mn in 2019. The market is estimated to grow with a CAGR of 22.2% from 2020-2027.
The growth of the market is driven by the factors such as, increasing adoption of IoT in healthcare and advantages of software as medical device (SaMD) .On the other hand, threat of data breach is likely to restraint the growth of market during the forecast years.
The North American Software as Medical Device market is expected to witness significant growth during the forecast period, due to factors such as the rising adoption of Internet of Things (IoT) in healthcare services for screening and monitoring of patient may offer vital growth opportunities for market growth. The US is among the top countries in the field of software as medical device. The presence of key players in the country and guidelines proposed by the US FDA for software as medical device are driving the growth of software as medical device in country.
The growth of the North American software as a medical device market is primarily driven by increasing adoption of smart health monitoring devices, transformation of digital healthcare, increasing number of chronic diseases, and support from the federal governments to implement digital tools in healthcare to cut down the costs and improve the quality of care. According to the US FDA, software has become an important part of all the products that are widely integrated into the digital healthcare platforms. The FDA also states that the use of software as medical device is continuously increasing in the US. The implementation of these devices is broadly seen across a range of digital platforms, including commercial off-shelf platforms, virtual networks, and medical device platforms. The International Medical Device Regulators Forum (IMDRF) is a voluntary group of medical device regulators that have come together to develop a harmonized regulation on medical devices. The US FDA specifies certain criteria to qualify software as medical devices.
North America Software as Medical Device Market Revenue and Forecast to 2027 (US$ Mn)
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
North America Software as Medical Device – Market Segmentation
By Device Type
- PCs/Laptops
- Smartphones/Tablets
- Wearable Devices
By Application
- Screening and Diagnosis
- Monitoring and Alerting
- Disease Management
By Deployment Type
- Cloud
- On-Premises
By Country
- US
- Canada
- Mexico
Company Profiles
- Velentium LLC
- Tietronix Software, Inc.
- S3 Connected Health
- Zühlke Group
- Science Group
- Inzentiz
- Cambridge Consultants Inc
- BrightInsight, Inc.
- CompliancePath
- Jabil Inc.
- Phillips-Medisize
- Pro4People Sp. Z.o.o
North America Software as a Medical Device Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 8,353.94 Million |
| Market Size by 2027 | US$ 39,854.20 Million |
| CAGR (2020 - 2027) | 22.2% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Device Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For