The Saudi Arabia endoscopy devices market size is projected to grow from US$ 470.79 million in 2022 to US$ 746.18 million by 2030; it is anticipated to record a CAGR of 5.9% from 2022 to 2030.
Market Insights and Analyst View:
An endoscope can be inserted into the openings of the body. For example, during a bronchoscopy, the scope is introduced through natural openings such as mouth, whereas in a sigmoidoscopy, it is inserted through the rectum. The device is used to visually examine internal organs and accordingly diagnose the medical conditions; it is employed in minimally invasive surgical procedures. The device usually comes with a light source. A medical procedure performed using any type of endoscope is called an endoscopy. Key factors driving the Saudi Arabia endoscopy devices market growth include the surging preference for minimally invasive surgeries and the increasing prevalence of cancer. However, risks of infections associated with endoscopic procedures hinder the market growth.
Growth Drivers and Restraints:
The major advantages of minimally invasive surgeries (MIS) over traditional surgical procedures include less post-operative pain, faster recovery, and reduced trauma and pain. Moreover, patients undergoing MIS need hospitalization for a relatively shorter span as minimal cuts or stitches are involved; moreover, patients need not visit the hospital frequently after the procedure. The growing preference for MIS significantly benefits the endoscopy devices market.
According to the article "Endoscopic Transnasal Approach to Petrous Apex," published in May 2022 in Frontiers Journal, endoscopy systems based on MIS methodology are employed to diagnose any disease or infection inside the body. Traditionally, most anatomical sites that are difficult to observe by microscope can be exposed through endoscopic-assisted procedures. Endoscopic devices adjust angles to aid better visibility into the surrounding anatomical structure through the natural human foramen. This can provide surgeons with an open visual field and an operation channel without retraction, significantly improving the overall surgical procedure. Additionally, healthcare providers, including surgeons and gastroenterologists, have increasingly embraced minimally invasive endoscopic techniques, which encourages them to invest in specialized training programs to perform these procedures effectively. For instance, in October 2022, MaxMoreSpine provided full hands-on training on the endoscopic spine surgery technique with the MaxMoreSpine system in Riyadh, Saudi Arabia. Thus, the growing expertise among physicians leads to a broad-scale adoption of minimally invasive endoscopic procedures across different medical specialties, thereby bolstering the demand for advanced endoscopy devices.
An increase in the prevalence of cancer drives the demand for endoscopy devices. These devices are used as vital tools for diagnosis (in biopsies), treatment, and monitoring during cancer treatments. Endoscopy procedures enable the early detection and diagnosis of various types of cancers, including esophageal, pancreatic, colorectal, and stomach cancer. Through the use of endoscopes and imaging technologies, healthcare providers can visualize the internal structures of organs to identify abnormalities, lesions, and early-stage tumors, thereby facilitating timely intervention and improved treatment outcomes.
On the other hand, the risk of infections associated with endoscopic procedures hampers the market growth. Endoscopes are generally compact and closed units operated by a broad spectrum of staff members, including endoscopists, nursing staff, technicians, anesthetists, and hospital attendants; patients awaiting various treatments; and many small and large equipment providers. Patients with a high viral load in their respiratory secretions can contaminate the endoscopic air and fomites with viruses that can survive for longer periods, placing uninfected patients and endoscopy staff at risk. Viral infections can be transmitted from person to person through direct contact or respiratory droplets; the generation of infected aerosols during endoscopy; or contact with contaminated endoscopic devices, accessories, and body fluids. Endoscopic procedures such as gastroscopy, colonoscopy, and endoscopic retrograde cholangiopancreatography are performed daily. Endoscopists work on the gastrointestinal lumen from a close distance; hence, they are exposed to several infections associated with respiratory tracts, oropharynx, and gastrointestinal systems. Along with professionals appointed in endoscopic units, medical staff from other departments are also susceptible to contracting a viral infection.
Contaminated endoscopes have been associated with many outbreaks of device-related nosocomial infections in the past. Although flexible endoscopes can be disinfected, they cannot be sterilized after use. This implies the risk of settlement of biofilm-producing species. Thus, the application of endoscopic devices in various procedures is limited by the risk of infections associated with endoscopic devices.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Saudi Arabia Endoscopy Devices Market: Strategic Insights
-
Market Size 2022
US$ 470.79 Million -
Market Size 2030
US$ 746.18 Million

Market Dynamics
- XXXXXXX
- XXXXXXX
- XXXXXXX
- XXXXXXX
- XXXXXXX
- XXXXXXX
- XXXXXXX
- XXXXXXX
- XXXXXXX
Regional Overview
- Saudi Arabia
Market Segmentation
 Product
Product
- Endoscopes
- Visualization Systems
- Accessories
- Other Endoscopy Devices
 Application
Application
- Gastroscopy
- Laparoscopy
- Arthroscopy
- Urology Endoscopy
- Bronchoscopy
- Laryngoscopy
- Otoscopy
 End User
End User
- Hospitals
- Ambulatory Surgical Centers
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The “Saudi Arabia endoscopy devices market” is segmented on the basis of the product, application, and end user. Based on product, the market is segmented into endoscopes, visualization systems, accessories, and other endoscopy devices. In terms of application, the Saudi Arabia endoscopy devices market is segmented into gastroscopy, laparoscopy, arthroscopy, urology endoscopy, bronchoscopy, laryngoscopy, otoscopy, and others. Based on end user, the market is segmented into hospitals, ambulatory surgical centers, and others.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Segmental Analysis:
The Saudi Arabia endoscopy devices market, by product, is segmented into endoscopes, visualization systems, accessories, and other endoscopy devices. The endoscopes segment held the largest share of the market in 2022, and the visualization systems segment is anticipated to register the highest CAGR in the market during 2022–2030. An endoscope is used in minimally invasive surgeries and can be inserted into the openings of the body. The device is commonly used to visually examine internal organs and accordingly diagnose medical conditions. However, some of these devices are also used for small surgical tasks due to their fine, flexible design, which helps reduce cost, time, and physical trauma that are associated with standard surgical procedures. Most of the endoscopes are similar in construction. The endoscopes are named as per the location of their use. For example, a nephroscope is used for the endoscopy of the kidney, an arthroscopy is used for joints, and a laparoscope for the endoscopy of the abdomen or pelvis. There are various types of endoscopes based on their structure, such as rigid, flexible, capsule, and robot-assisted endoscopes.
Saudi Arabia Endoscopy Devices Market, by Products – 2022 and 2030
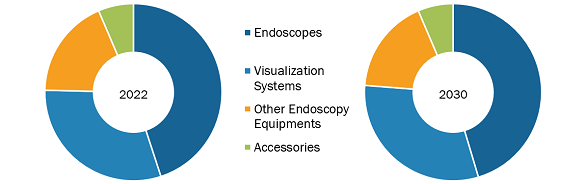
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Based on application, the Saudi Arabia endoscopy devices market is segmented into gastroscopy, laparoscopy, arthroscopy, urology endoscopy, bronchoscopy, laryngoscopy, otoscopy, and others. The gastroscopy segment held the largest share of the market in 2022. However, the laparoscopy segment is expected to register the highest CAGR in the market from 2022 to 2030. Gastroscopy, also known as upper endoscopy, involves examining the upper gastrointestinal tract—the stomach, esophagus, and duodenum (the beginning part of the small intestine). It is used to diagnose conditions such as ulcers, tumors, inflammation, and gastroesophageal reflux disease (GERD). Laparoscopy, commonly referred to as minimally invasive surgery, involves using a laparoscope to visualize the abdominal or pelvic cavities for diagnostic or surgical purposes. This approach offers reduced incision size, faster recovery, and lower complication risks compared to traditional open surgeries.
Based on end user, the Saudi Arabia endoscopy devices market is segmented into hospitals, ambulatory surgical centers, and others. The hospitals segment held the largest share of the market in 2022, and the same segment is expected to register the highest CAGR in the market from 2022 to 2030. Hospitals encompass a wide spectrum of medical facilities, ranging from community hospitals to large academic medical centers. They serve as major end users of endoscopy devices. The demand for endoscopy devices within hospital settings is driven by factors such as comprehensive care delivery, inpatient and outpatient services, and advanced procedures and interventions.
Country Analysis:
The endoscopy devices market in Saudi Arabia is undergoing significant transformation owing to factors such as developments in healthcare infrastructure, rapidly growing medical tourism, and acceptance and adoption of advanced medical technologies. The private sector with rising investments has been playing an essential role in the Saudi healthcare system. Efforts from this sector help in the timely delivery of healthcare services to numerous patients. The Saudi Vision 2030 program of the government of Saudi Arabia aims to reduce its dependence on the oil & gas sector and diversify its economy by promoting the growth of other sectors, including healthcare. Moreover, with a projected investment of US$ 13.8 billion by 2030 to improve medical facilities, the adoption of advanced surgical equipment and medical devices is likely to increase in the country in the future.
The Saudi Food and Drug Authority (SFDA) plays a pivotal role in regulating medical devices, including endoscopy devices, ensuring adherence to international standards and certifications, thus fostering confidence in the safety and efficacy of endoscopic technologies. Similarly, Saudi Arabia's commitment to enhancing healthcare infrastructure, including efforts such as the construction of state-of-the-art hospitals, specialty clinics, and medical centers, triggers the demand for advanced endoscopy devices that support a wide range of diagnostic and interventional services.
Industry Developments and Future Opportunities:
Various initiatives taken by key players operating in the endoscopy devices market are listed below:
- In October 2023, Olympus Corp announced the launch of the next-generation EVIS X1 endoscopy system in the market. The company had plans to showcase and demonstrate the product during October 22–24, 2023, at the yearly meeting of the American College of Gastroenterology (ACG) in Vancouver, Canada. With the amazing new tools available for GI tract visualization, doctors will be able to make better observations and assist their patients more effectively.
- In October 2023, Olympus announced the release of its EU-ME3 endoscopic ultrasound processing platform. The EU-ME3 meets medical practitioners’ demands for high-quality images during endoscopic ultrasounds. During this fiscal year, the company intends to launch the system in Europe, the Middle East, Africa, sections of Asia, and Oceania.
- In September 2023, Ambu expanded its gastroenterology portfolio with the announcement of the Ambu aScope Gastro Large and Ambu aBox 2, two new larger gastroscopy solutions that will be available in Europe. In addition to being the first gastroscope in the world with a 4.2 mm operating channel, which enables gastroenterologists to achieve strong suction performance during procedures in ICUs and endoscopy units, the Ambu aScope Gastro Large is the first endoscope ever manufactured of bioplastic materials.
- In February 2023, Boston Scientific Corp received FDA approval for its LithoVue Elite Single-Use Digital Flexible Ureteroscope System. It is the first ureteroscope system that can monitor intrarenal pressure in real time during ureteroscopy procedures. The LithoVue Elite system comprises the StoneSmart Connect Console, which has been upgraded to offer superior image quality, control features, and streamlined integration.
- In September 2022, Medtronic plc received US FDA approval for its Nexpowder endoscopic hemostasis system. The hemostasis system is supplied worldwide by Medtronic and is separately developed by NEXTBIOMEDICAL CO., LTD (Korea). The system is incorporated with a catheter with patented powder-coating technology, which uses noncontact, nonthermal, and nontraumatic hemostatic powder sprayed on the component.
Competitive Landscape and Key Companies:
Boston Scientific Corp, Medtronic Plc, Stryker Corp, Johnson & Johnson, Karl Storz SE & Co KG, Olympus Corp, Ambu AS, Conmed Corp, B Braun SE, and PENTAX Medical are among the prominent players operating in the Saudi Arabia endoscopy devices market. These companies focus on new technologies, advancements in existing products, and geographic expansions to meet the growing consumer demand worldwide and increase their product range in specialty portfolios.
Saudi Arabia Endoscopy Devices Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 470.79 Million |
| Market Size by 2030 | US$ 746.18 Million |
| CAGR (2022 - 2030) | 5.9% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
Saudi Arabia
|
| Market leaders and key company profiles |
|
Frequently Asked Questions
Which application segment dominates the endoscopy devices market?
Which end user segment dominates the endoscopy devices market?
What was the estimated endoscopy devices market size in 2022?
What are the growth estimates for the endoscopy devices market till 2030?
What are endoscopy devices?
Which product segment dominates the endoscopy devices market?
What factors drive the Saudi Arabia endoscopy devices market?
Who are the major players in the endoscopy devices market?
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





















 Get Free Sample For
Get Free Sample For