Specimen Validity Testing Market Analysis and Forecast by Size, Share, Growth, Trends 2031
Specimen Validity Testing Market Size and Forecasts (2021 - 2031), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Product (Reagents & Assay Kits, Calibrators & Controls, Disposables); Type (Laboratory Testing, Rapid/PoC Testing); End User (Pain Management Centers, Workplaces, Criminal Justice & Law Enforcement Agencies, Drug Rehabilitation Centers, Drug Screening Laboratories, Others) and Geography , and Geography (North America, Europe, Asia Pacific, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Apr 2026
- Report Code : TIPBT00002235
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
The Specimen Validity Testing Market size is expected to reach US$ 5.03 billion by 2031. The market is anticipated to register a CAGR of 6.8% during 2025–2031.
The report is categorized by Product (Reagents & Assay Kits, Calibrators & Controls, Disposables) and further analyzes the market based on Type (Laboratory Testing, Rapid/PoC Testing). It also examines the market by End User (Pain Management Centers, Workplaces, Criminal Justice & Law Enforcement Agencies, Drug Rehabilitation Centers, Drug Screening Laboratories).A comprehensive breakdown is provided at global, regional, and country levels for each of these key segments.
The report includes market size and forecasts across all segments, presenting values in USD. It also delivers key statistics on the current market status of leading players, along with insights into prevailing market trends and emerging opportunities.
Purpose of the Report
The report Specimen Validity Testing Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
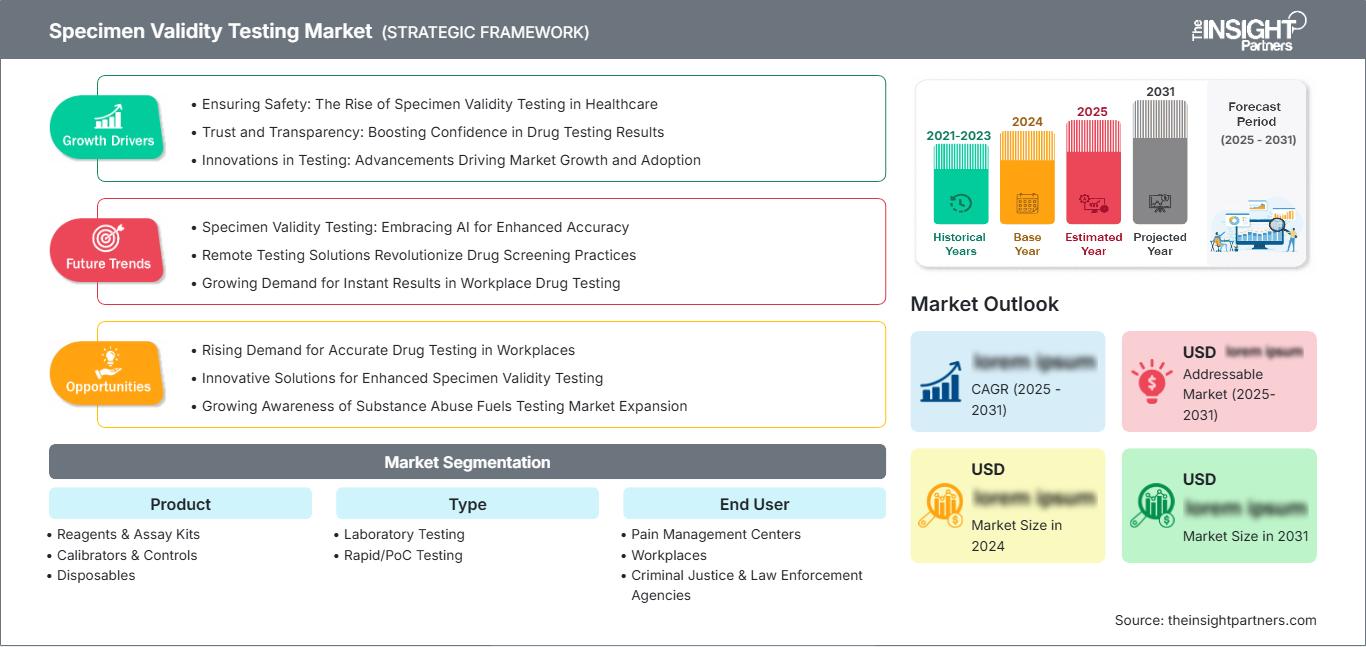
Specimen Validity Testing Market Segmentation Product
- Reagents & Assay Kits
- Calibrators & Controls
- Disposables
Type
- Laboratory Testing
- Rapid/PoC Testing
End User
- Pain Management Centers
- Workplaces
- Criminal Justice & Law Enforcement Agencies
- Drug Rehabilitation Centers
- Drug Screening Laboratories
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONSpecimen Validity Testing Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Specimen Validity Testing Market Growth Drivers
- Ensuring Safety: The Rise of Specimen Validity Testing in Healthcare
- Trust and Transparency: Boosting Confidence in Drug Testing Results
- Innovations in Testing: Advancements Driving Market Growth and Adoption
Specimen Validity Testing Market Future Trends
- Specimen Validity Testing: Embracing AI for Enhanced Accuracy
- Remote Testing Solutions Revolutionize Drug Screening Practices
- Growing Demand for Instant Results in Workplace Drug Testing
Specimen Validity Testing Market Opportunities
- Rising Demand for Accurate Drug Testing in Workplaces
- Innovative Solutions for Enhanced Specimen Validity Testing
- Growing Awareness of Substance Abuse Fuels Testing Market Expansion
Specimen Validity Testing Market Regional Insights
The regional trends and factors influencing the Specimen Validity Testing Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Specimen Validity Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Specimen Validity Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX Billion |
| Market Size by 2031 | US$ 5.03 Billion |
| Global CAGR (2025 - 2031) | 6.8% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Specimen Validity Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Specimen Validity Testing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Specimen Validity Testing Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Specimen Validity Testing Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Specimen Validity Testing Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Related Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For