The Europe clinical trial supplies market is expected to reach US$ 938.54 million by 2027 from US$ 550.28 million in 2019; it is estimated to grow at a CAGR of 7.0% from 2020 to 2027.
The growth of the market is attributed to increase in number of clinical trials and rise in R&D expenditures by the pharmaceutical & biopharmaceutical companies. However, rising cost of drug development and clinical trials is expected to restrain the growth of the market during the forecast years.
The clinical trial is an investigational study that defines whether a medical approach, therapy, or device is effective, safe, and useful for human applications. These studies help to find which therapeutic approach is best for certain diseases. Clinical trial supplies management is necessary for evading overproduction, oversupply, and inventory expiration. With the increasing costs of drug discovery, clinical trial supplies are obtaining more importance. A rise in outsourcing activities by pharmaceutical companies has been witnessed during recent years. The major factors driving the outsourcing activities by companies include cost cutting, need for innovations, increased speed and agility, and access to specialized knowledge and technologies. Hence, with an increase in the R&D expenditure, the need for clinical trial outsourcing is increasing. Furthermore, the collaborative research between the research & development team of the sponsoring company as well as contract developer is a new trend being employed in the market. In addition, the emerging trend of outsourcing has led to rise in development of drug candidates and clinical trials by pharmaceutical and biotechnology companies, which is also expected to account for the growth of the market over the coming years. Also, factors such as increasing R&D expenditures in the pharmaceutical & biopharmaceutical companies and rising number of clinical trials will bolster the market growth.
Since the surge of COVID-19 cases, the pharmaceutical industry is facing a decrease in production capabilities, resulting in drug shortages. Moreover, diversion of resources from drug development to coronavirus treatment is likely to hamper overall productivity of the drug development for a short period of span. Various pharmaceutical companies made decisions to postpone the ongoing clinical trials due to disrupted supply chains and threat of the virus. However, the procedures and practices have now re-initiated due to economic losses in the commercial sector in North America. Thus, the COVID-19 pandemic had a positive as well as negative impact on the Europe clinical trial supplies market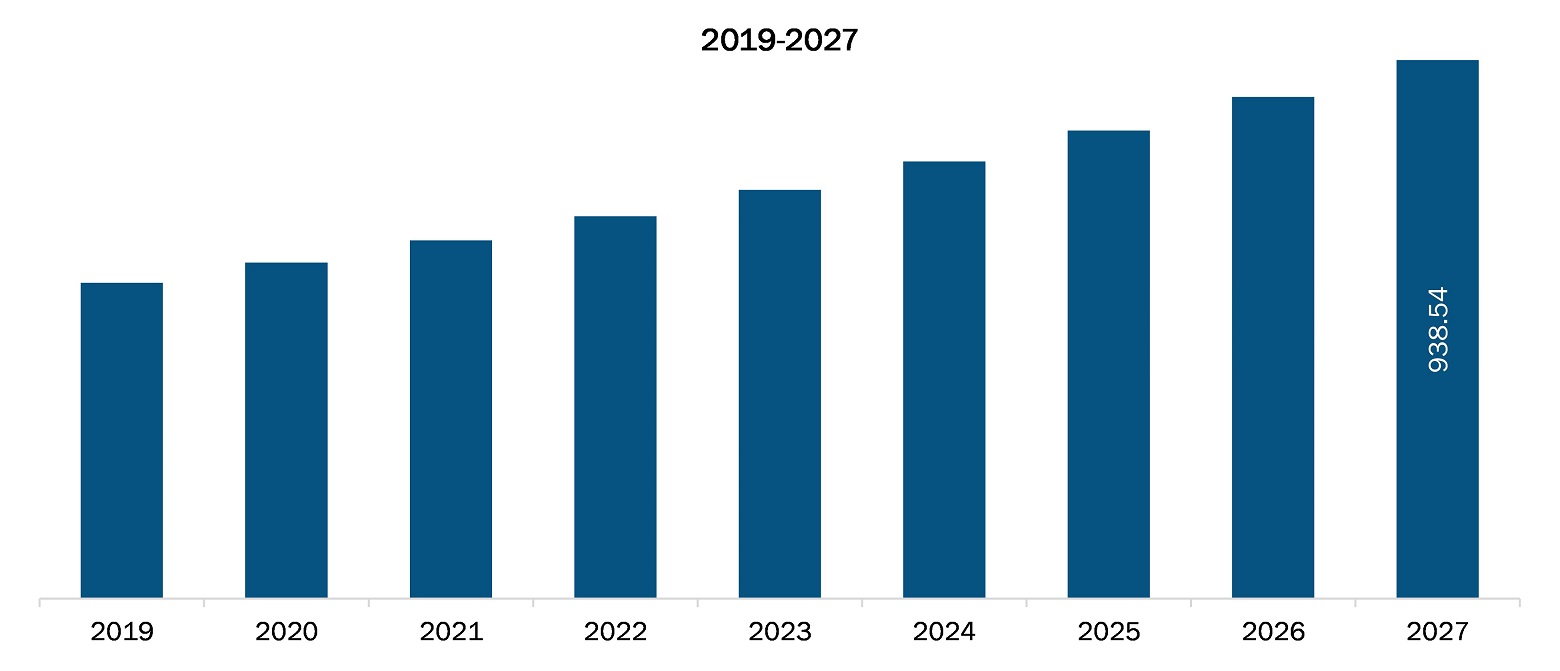
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
EUROPE CLINICAL TRIAL SUPPLIES MARKET SEGMENTATION
By Product & Service
- Manufacturing
- Packaging & Labelling
- Logistics & Distribution
By Stage
- Phase I
- Phase II
- Phase III
- Bioequivalence Studies
By Drug Type
- Small-molecule Drugs
- Biologic Drugs
By Application
- Oncology
- Cardiovascular Diseases
- Neurological Disorders
- Respiratory Disorders
- Others
By Country
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
Company Profiles
- THERMO FISHER SCIENTIFIC INC.
- UDG Healthcare plc
- Catalent Inc.
- pci Pharma Services
- Almac Group
- PAREXEL INTERNATIONAL CORPORATION
- Biocair
- Owens & Minor Inc
- Rubicon Research Pvt. Ltd
- CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC.
- KLIFO

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product & Service, Stage, Drug Type, and Application

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
UK, Germany, France, Italy, Russia
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 Europe Clinical Trial Supplies Market – By Product & Service
1.3.2 Europe Clinical Trial Supplies Market – By Stage
1.3.3 Europe Clinical Trial Supplies Market – By Drug Type
1.3.4 Europe Clinical Trial Supplies Market – By Application
1.3.5 Europe Clinical Trial Supplies Market – By Country
2. Clinical Trial Supplies Market – Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. Europe Clinical Trial Supplies Market– Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 Europe – PEST Analysis
4.3 Expert Opinion
5. Clinical Trial Supplies Market – Key Market Dynamics
5.1 Market Drivers
5.1.1 Increasing Pharmaceutical and Biopharmaceutical R&D Expenditures
5.1.2 Higher Cost of Drug Development Process in Developed Countries
5.2 Market Restraints
5.2.1 Rising Cost of Drug Development and Clinical Trials
5.2.2 Challenges for Clinical Trials Due to Negative Impact of Corona Virus
5.3 Future Trends
5.3.1 Growing Biosimilars and Biologics Market
5.4 Impact analysis
6. Clinical Trial Supplies Market – Europe Analysis
6.1 Europe Clinical Trial Supplies Market Revenue Forecast and Analysis
7. Clinical Trial Supplies Market Analysis – By Product and Service
7.1 Overview
7.2 Clinical Trial Supplies Market Revenue Share, by Product and Service (2019 and 2027)
7.3 Logistics & Distribution
7.3.1 Overview
7.3.2 Logistics & Distribution: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
7.4 Manufacturing
7.4.1 Overview
7.4.2 Manufacturing: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
7.5 Packaging & Labelling
7.5.1 Overview
7.5.2 Packaging & Labelling: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
8. Clinical Trial Supplies Market – By Stage
8.1 Overview
8.2 Clinical Trial Supplies Market, by Stage, 2019 and 2027 (%)
8.3 Phase III
8.3.1 Overview
8.3.2 Phase III: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
8.4 Phase II
8.4.1 Overview
8.4.2 Phase II: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
8.5 Bioequivalence Studies
8.5.1 Overview
8.5.2 Bioequivalence Studies: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
8.6 Phase I
8.6.1 Overview
8.6.2 Phase I: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
9. Clinical Trial Supplies Market – By Drug Type
9.1 Overview
9.2 Clinical Trial Supplies Market, by Drug Type 2019 and 2027 (%)
9.3 Small-molecule Drugs
9.3.1 Overview
9.3.2 Small-molecule Drugs: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
9.4 Biologic Drugs
9.4.1 Overview
9.4.2 Biologic Drugs: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
10. Clinical Trial Supplies Market – By Application
10.1 Overview
10.2 Clinical Trial Supplies Market, by Application 2019 and 2027 (%)
10.3 Oncology
10.3.1 Overview
10.3.2 Oncology: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
10.4 Cardiovascular Diseases
10.4.1 Overview
10.4.2 Cardiovascular Diseases: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
10.5 Neurological Disorders
10.5.1 Overview
10.5.2 Neurological Disorders: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
10.6 Respiratory Disorders
10.6.1 Overview
10.6.2 Respiratory Disorders: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
10.7 Others
10.7.1 Overview
10.7.2 Others: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
11. Clinical Trial Supplies Market – Europe Analysis
11.1 Europe: Clinical Trial Supplies Market
11.1.1 Overview
11.1.2 Europe: Clinical Trial Supplies Market, by Country, 2018 & 2027 (%)
11.1.3 Germany: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.3.1 Germany: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.3.2 Germany: Clinical Trial Supplies Market, by Product & Service, 2018–2027 (USD Million)
11.1.3.3 Germany: Clinical Trial Supplies Market, by Stage, 2018–2027 (USD Million)
11.1.3.4 Germany: Clinical Trial Supplies Market, by Drug Type, 2018–2027 (USD Million)
11.1.3.5 Germany: Clinical Trial Supplies Market, by Application, 2018–2027 (USD Million)
11.1.4 UK: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.4.1 UK: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.4.2 UK: Clinical Trial Supplies Market, by Product & Service, 2018–2027 (USD Million)
11.1.4.3 UK: Clinical Trial Supplies Market, by Stage, 2018–2027 (USD Million)
11.1.4.4 UK: Clinical Trial Supplies Market, by Drug Type, 2018–2027 (USD Million)
11.1.4.5 UK: Clinical Trial Supplies Market, by Application, 2018–2027 (USD Million)
11.1.5 France: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.5.1 France: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.5.2 France: Clinical Trial Supplies Market, by Product & Service, 2018–2027 (USD Million)
11.1.5.3 France: Clinical Trial Supplies Market, by Stage, 2018–2027 (USD Million)
11.1.5.4 France: Clinical Trial Supplies Market, by Drug Type, 2018–2027 (USD Million)
11.1.5.5 France: Clinical Trial Supplies Market, by Application, 2018–2027 (USD Million)
11.1.6 Spain: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.6.1 Spain: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.6.2 Spain: Clinical Trial Supplies Market, by Product & Service, 2018–2027 (USD Million)
11.1.6.3 Spain: Clinical Trial Supplies Market, by Stage, 2018–2027 (USD Million)
11.1.6.4 Spain: Clinical Trial Supplies Market, by Drug Type, 2018–2027 (USD Million)
11.1.6.5 Spain: Clinical Trial Supplies Market, by Application, 2018–2027 (USD Million)
11.1.7 Italy: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.7.1 Italy: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
11.1.7.2 Italy: Clinical Trial Supplies Market, by Product & Service, 2018–2027 (USD Million)
11.1.7.3 Italy: Clinical Trial Supplies Market, by Stage, 2018–2027 (USD Million)
11.1.7.4 Italy: Clinical Trial Supplies Market, by Drug Type, 2018–2027 (USD Million)
11.1.7.5 Italy: Clinical Trial Supplies Market, by Application, 2018–2027 (USD Million)
12. Impact of COVID-19 Pandemic on Europe Clinical Trial Supplies Market
12.1 Europe: Impact assessment of COVID-19 Pandemic
13. Clinical Trials Supplies Market – Industry Landscape
13.1 Overview
13.2 Organic Developments
13.2.1 Overview
13.3 Inorganic Developments
13.3.1 Overview
14. Clinical Trial Supplies Market – Key Company Profiles
14.1 THERMO FISHER SCIENTIFIC INC.
14.1.1 Key Facts
14.1.2 Business Description
14.1.3 Products and Services
14.1.4 Financial Overview
14.1.5 SWOT Analysis
14.1.6 Key Developments
14.2 UDG Healthcare plc
14.2.1 Key Facts
14.2.2 Business Description
14.2.3 Products and Services
14.2.4 Financial Overview
14.2.5 SWOT Analysis
14.2.6 Key Developments
14.3 Catalent Inc.
14.3.1 Key Facts
14.3.2 Business Description
14.3.3 Products and Services
14.3.4 Financial Overview
14.3.5 SWOT Analysis
14.3.6 Key Developments
14.4 pci Pharma Services
14.4.1 Key Facts
14.4.2 Business Description
14.4.3 Products and Services
14.4.4 Financial Overview
14.4.5 SWOT Analysis
14.4.6 Key Developments
14.5 Almac Group
14.5.1 Key Facts
14.5.2 Business Description
14.5.3 Products and Services
14.5.4 Financial Overview
14.5.5 SWOT Analysis
14.5.6 Key Developments
14.6 PAREXEL INTERNATIONAL CORPORATION
14.6.1 Key Facts
14.6.2 Business Description
14.6.3 Products and Services
14.6.4 Financial Overview
14.6.5 SWOT Analysis
14.6.6 Key Developments
14.7 Biocair
14.7.1 Key Facts
14.7.2 Business Description
14.7.3 Products and Services
14.7.4 Financial Overview
14.7.5 SWOT Analysis
14.7.6 Key Developments
14.8 Owens & Minor Inc.
14.8.1 Key Facts
14.8.2 Business Description
14.8.3 Products and Services
14.8.4 Financial Overview
14.8.5 SWOT Analysis
14.8.6 Key Developments
14.9 KLIFO
14.9.1 Key Facts
14.9.2 Business Description
14.9.3 Products and Services
14.9.4 Financial Overview
14.9.5 SWOT Analysis
14.9.6 Key Developments
14.10 CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC.
14.10.1 Key Facts
14.10.2 Business Description
14.10.3 Products and Services
14.10.4 Financial Overview
14.10.5 SWOT Analysis
14.10.6 Key Developments
15. Appendix
15.1 About The Insight Partners
15.2 Glossary of Terms
LIST OF TABLES
Table 1. Germany Clinical Trial Supplies Market, by Product & Service – Revenue and Forecast to 2027 (USD Million)
Table 2. Germany Clinical Trial Supplies Market, by Stage – Revenue and Forecast to 2027 (USD Million)
Table 3. Germany Clinical Trial Supplies Market, by Drug Type – Revenue and Forecast to 2027 (USD Million)
Table 4. Germany Clinical Trial Supplies Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 5. UK Clinical Trial Supplies Market, by Product & Service – Revenue and Forecast to 2027 (USD Million)
Table 6. UK Clinical Trial Supplies Market, by Stage – Revenue and Forecast to 2027 (USD Million)
Table 7. UK Clinical Trial Supplies Market, by Drug Type – Revenue and Forecast to 2027 (USD Million)
Table 8. UK Clinical Trial Supplies Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 9. France Clinical Trial Supplies Market, by Product & Service – Revenue and Forecast to 2027 (USD Million)
Table 10. France Clinical Trial Supplies Market, by Stage – Revenue and Forecast to 2027 (USD Million)
Table 11. France Clinical Trial Supplies Market, by Drug Type – Revenue and Forecast to 2027 (USD Million)
Table 12. France Clinical Trial Supplies Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 13. Spain Clinical Trial Supplies Market, by Product & Service – Revenue and Forecast to 2027 (USD Million)
Table 14. Spain Clinical Trial Supplies Market, by Stage – Revenue and Forecast to 2027 (USD Million)
Table 15. Spain Clinical Trial Supplies Market, by Drug Type – Revenue and Forecast to 2027 (USD Million)
Table 16. Spain Clinical Trial Supplies Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 17. Italy Clinical Trial Supplies Market, by Product & Service – Revenue and Forecast to 2027 (USD Million)
Table 18. Italy Clinical Trial Supplies Market, by Stage – Revenue and Forecast to 2027 (USD Million)
Table 19. Italy Clinical Trial Supplies Market, by Drug Type – Revenue and Forecast to 2027 (USD Million)
Table 20. Italy Clinical Trial Supplies Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 21. Organic Developments Done by Companies
Table 22. Inorganic Developments Done by Companies
Table 23. Glossary of Terms, Clinical Trial Supplies Market
LIST OF FIGURES
Figure 1. Clinical Trial Supplies Market Segmentation
Figure 2. Clinical Trial Supplies Segmentation, By Country
Figure 3. Europe Clinical Trial Supplies Market Overview
Figure 4. Logistics & Distribution Segment Held Largest Share of Product & Service Segment in Clinical Trial Supplies Market
Figure 5. Phase III Segment Held Largest Share of Stage Segment in Clinical Trial Supplies Market
Figure 6. Small-molecule Drugs Segment Held Largest Share of Drug type Segment in Clinical Trial Supplies Market
Figure 7. Oncology Segment Held Largest Share of Application Segment in Clinical Trial Supplies Market
Figure 8. Europe Clinical Trial Supplies Market- Leading Country Markets (US$ Million)
Figure 9. Europe PEST Analysis
Figure 10. Clinical Trial Supplies Market Impact Analysis of Drivers and Restraints
Figure 11. Europe Clinical Trial Supplies Market – Revenue Forecast and Analysis – 2019- 2027
Figure 12. Clinical Trial Supplies Market Revenue Share, by Product and Service (2019 and 2027)
Figure 13. Logistics & Distribution: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 14. Manufacturing: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 15. Packaging & Labelling: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 16. Clinical Trial Supplies Market, by Stage 2019 and 2027 (%)
Figure 17. Phase III: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 18. Phase II: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 19. Bioequivalence Studies: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 20. Phase I: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 21. Clinical Trial Supplies Market, by Drug Type, 2019 and 2027 (%)
Figure 22. Small-molecule Drugs: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 23. Biologics Drugs: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 24. Clinical Trial Supplies Market, by Application, 2019 and 2027 (%)
Figure 25. Oncology: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 26. Cardiovascular Diseases: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 27. Neurological Disorders: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 28. Respiratory Disorders: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 29. Others: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (US$ Million)
Figure 30. Europe: Clinical Trial Supplies Market, by Key Country – Revenue (2019) (USD Million)
Figure 31. Europe: Clinical Trial Supplies Market, by Country, 2018 & 2027 (%)
Figure 32. Germany: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
Figure 33. UK: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
Figure 34. France: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
Figure 35. Spain: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
Figure 36. Italy: Clinical Trial Supplies Market – Revenue and Forecast to 2027 (USD Million)
Figure 37. Impact of COVID-19 Pandemic in European Country Markets
- THERMO FISHER SCIENTIFIC INC.
- UDG Healthcare plc
- Catalent Inc.
- pci Pharma Services
- Almac Group
- PAREXEL INTERNATIONAL CORPORATION
- Biocair
- Owens & Minor Inc
- Rubicon Research Pvt. Ltd
- CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC.
- KLIFO
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to Europe Clinical Trials Supplies Market

May 2021
Aquatic Veterinary Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Diagnostics and Treatments), Species (Fish, Crustaceans, Mollusca, and Others), Disease Source (Bacteria, Viruses, Parasites, and Others), Route of Administration (Water Medication, Medicated Feed, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

May 2021
Sickle Cell Disease Treatment Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis By Treatment (Generic Drugs and Originators), Route of Administration (Oral and Parenteral), and Distribution Channel (Direct Tender, Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, and Others)

May 2021
Malaria and Sickle Cell Disease Treatment Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Treatment [Malaria and Sickle Cell Disease (SCD)], Route of Administration (Oral and Parenteral), and Distribution Channel (Direct Tender, Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, and Others)

May 2021
Pharmaceutical Contract Sales Organizations Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Services (Commercial Services and Non-Commercial Services), Modules (Syndicated Modules and Dedicated Modules), Therapeutic Area (Cardiovascular Disorders, Oncology, Metabolic Disorders, Neurology, Orthopedic Diseases, Infectious Diseases, and Others), End User (Biopharmaceutical Companies and Pharmaceutical Companies), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

May 2021
Breast Cancer Therapeutics Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Drug Therapy [Targeted Drug Therapy (Abemaciclib, Ado-Trastuzumab Emtansine, Palbociclib, Trastuzumab, and Other Target Drug Therapies), Hormonal Drug Therapy (Selective Estrogen Receptor Modulators, Aromatase Inhibitors, and Selective Estrogen Receptor Downregulators), Chemotherapy, and Immunotherapy/Biological Therapy], Breast Cancer Type (Hormone Receptor, HER2+, and Triple-Negative Breast Cancer), Distribution Channel (Hospital Pharmacies, Drug Stores and Retail Pharmacies, and Online Pharmacies), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

May 2021
Radioactive Tracer Market
Size and Forecast to 2030 - Global Analysis by Tracer Type [Technetium-99m & Tc-97m, Iodine-131, Iron-59, Lutetium-171, Rubidium (Rb-82) Chloride & Ammonia (N-13), Scandium-46, Seaborgium-269, Hassium-269, Gallium Citrate Ga 67, Prostate-Specific Membrane Antigen (PSMA) (Ga-68), FDDNP (F-18) & FDOPA (F-18), Phosphorus-32 & Chromium-51, Thallium-201, F-18 FDG, F-18 FAPI, Ga-68 FAPI, F-18 PSMA, DOTATOC/DOTANOC/DOTATATE (Ga-68), and Others], Test Type (PET, SPECT, and Others), Application (Oncology, Pulmonary, Neurology, Cardiology, and Others) End User (Hospitals & Clinics, Diagnostic Centers, Academic & Research Institutes, And Others), and Geography

May 2021
Glioma Treatment Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Disease (Astrocytoma, Oligoastrocytoma, and Oligodendroglioma), Treatment Type (Surgery, Chemotherapy, Radiation Therapy, and Others), Grade (Low Grade and High Grade), End User (Hospital & Clinics and Ambulatory Surgical Center), and Geography

May 2021
Hyperpigmentation Disorder Treatment Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Treatment Type (Cosmeceutical, Light or Laser Therapy, Microdermabrasion, Chemical Peels, Cryotherapy, and Others), Condition (Melasma, Solar Lentigines, Post-Inflammatory Hyperpigmentation, and Others), End User (Hospitals, Dermatology Centers, and Others), and Geography (North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa)