Vital Signs Monitoring Devices Market Key Companies and SWOT Analysis by 2030
Vital Signs Monitoring Devices Market Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Product (Pulse Oximeters, Blood Pressure Monitors, Temperature Monitoring Devices, and Glucose Monitoring Devices), End User (Hospitals and Clinics, Ambulatory Care Centers, Home Healthcare, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Historic Data: 2020-2021 | Base Year: 2022 | Forecast Period: 2023-2030- Report Date : Sep 2023
- Report Code : TIPHE100001258
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 223
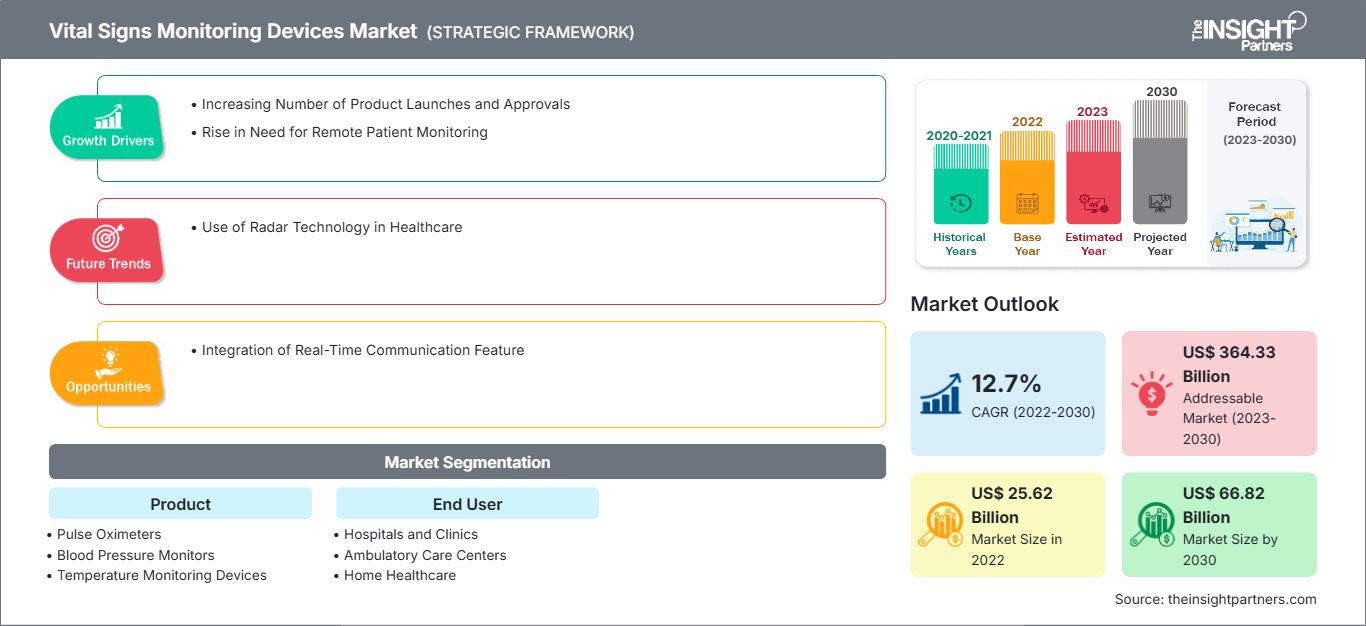
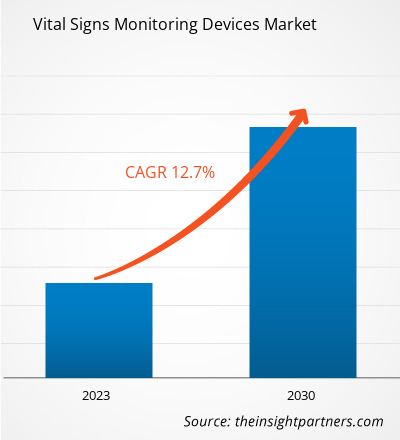
[Research Report] The vital signs monitoring devices market size was valued at US$ 25,617.24 million in 2022 and is expected to reach US$ 66,824.03 million by 2030. It is estimated to grow at a CAGR of 12.7% from 2022 to 2030
Market Insights and Analyst View:
The growing adoption of remote patient monitoring drives the growth of the vital signs monitoring devices market. During the COVID-19 pandemic, the initiation of telemedicine has leveraged the growth of home healthcare. In addition, the growing awareness about using pulse oximeters, thermometers, and blood pressure monitors to keep track of vitals as precautionary measures propelled the demand for these devices. A surge in preference for home healthcare—largely driven by the growing geriatric population and rising awareness about healthy and fit lifestyles—is another vital factor boosting the vital signs monitoring devices market.
Growth Drivers and Challenges:
In the global vital signs monitoring devices market companies are designing, developing, and upgrading their existing products by harnessing ongoing advancements in medical technology. Frequent product launches significantly contribute to market growth by encouraging players to compete with each other by developing innovative products and obtaining regulatory approvals for their products. A few of the recent product launches and approvals that have significantly contributed to the market growth are mentioned below.
- In June 2023, Masimo received US Food and Drug Administration (FDA) approval for its Radius VSM, a continuous, multi-parameter vital signs monitor. The device was developed to allow physicians to measure physiological parameters such as blood pressure, temperature, respiration rate, ECG, and oxygen. The Masimo SET pulse oximeter integrated into Radius VSM allows it to keep a check on the oxygen levels of patients. In addition, Masio Radius VSM is a self-operated device, or it can also be used by connecting it wirelessly to Masimo bedside monitors that include Root and the Masimo Hospital Automation platform.
- In April 2023, Honeywell introduced a real-time health monitoring system to capture patient’s vital signs in hospitals and remote setups. The sensing technology incorporated in the system monitors vital signs through a skin patch and instantaneously notifies healthcare providers on their mobile devices and an online dashboard. Through this innovation, the company can aid improvements in patient care at home, hospitals, and ambulatory surgical centers.
- In June 2022, GE Healthcare announced the launch of Portrait Mobile, a product developed by combining wireless patient-worn sensors and a smartphone-style monitor. People wearing this monitoring device can move freely without any location restrictions. In August 2023, GE Healthcare received FDA approval for the Portrait Mobile and 510(k) approval for its Carescape Canvas patient monitoring platform.
- In April 2021, Oxehealth Service won FDA approval for its vital sign software, which can monitor heart rate and breathing with an overhead camera attached to the device. The product is suitable for end users in nursing homes and long-term care facilities.
- In January 2020, BioIntelliSense, Inc. announced the commercial launch of its Data-as-a-Service (DaaS) platform. It also announced receiving FDA 510(k) clearance for its BioSticker, an on-body sensor for remote care devices. Further, in July 2020, BioIntelliSense, Inc. collaborated with Royal Philips to integrate BioSticker into Philips’s remote patient monitoring devices.
On the other hand, the recall rate of vital signs monitoring devices has increased in recent years. A few major players in the vital signs monitoring devices market have executed product recalls in the last several years. Regulatory authorities have raised concerns about product recalls showcasing players' shortcomings and affecting their brand image. Thus, product recalls by companies and regulatory authorities are hindering the growth of the vital signs monitoring devices market.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONVital Signs Monitoring Devices Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The vital signs monitoring devices market is segmented on the basis of product and end user. The market, based on product, is subsegmented into pulse oximeters, temperature monitoring devices, blood pressure monitors, and glucose monitoring devices. On the basis of end user, the market is categorized into hospitals and clinics, ambulatory care centers, home healthcare, and others. Based on geography, the market is divided into North America (US, Canada, and Mexico), Europe (UK, Germany, France, Italy, Spain, and Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and Rest of Asia Pacific), Middle East & Africa (South Africa, Saudi Arabia, UAE, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America).
Segmental Analysis:
Based on product, the glucose monitors segment accounted for the largest share of the vital signs monitoring devices market in 2022. The pulse oximeters segment is estimated to register the fastest CAGR of during 2022–2030. Glucose monitoring devices are used to diagnose both hyperglycemic and hypoglycemic conditions in diabetic patients. These devices allow patients and clinicians to detect high or low blood glucose levels, further allowing therapy modifications and protecting patients by promptly confirming acute hypoglycemia or hyperglycemia. The technology contributes to patients’ knowledge about diabetes and its management.
Pulse oximeters are electronic devices used to determine the saturation of oxygen carried in red blood cells. The growth of the market for pulse oximeters is majorly driven by a rising prevalence of cardiovascular diseases and respiratory diseases, an increase in demand for minimally invasive surgeries, and newer developments in these devices. Pulse oximeters are available in table-top and handheld models along with accessories.
On the basis of end user, the market is categorized into hospitals and clinics, ambulatory care centers, home healthcare, and others. The hospitals and clinics segment held the largest market share in 2022. The home healthcare segment is estimated to register the fastest CAGR during 2022–2030. The increasing number of hospitals and clinics, surging preference for home healthcare settings, and rising R&D in medical devices are the key factors contributing to the growth of the vital signs monitoring devices market.
Regional Analysis:
Based on geography, thevital signs monitoring devices market is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. North America is the largest contributor to the growth of the market, and Europe is expected to register the fastest CAGR during 2022–2030. The market growth in North America is attributed to an extensive rise in respiratory diseases, a large pool of diabetic population, and an increase in awareness of respiratory diseases among the population. In Europe, the vital signs monitoring devices market is driven by factors such as the rising incidences of chronic diseases, an increasing number of product launches, growing investments for product developments, and a surge in the aging population.
Industry Developments and Future Opportunities:
Various initiatives by key players operating in the vital signs monitoring devices market are listed below:
- In August 2023, GE HealthCare unveiled its smartphone-sized Portrait Mobile to continuously monitor vital signs of patients using wearable sensors with a wireless protocol. This device does not require patients to continuously lie on hospital beds for vitals monitoring. Portrait Mobile features an innovative measurement technology to capture respiration rate, one of the most sensitive vital signs for early patient deterioration, continuously, accurately, and reliably.
- In May 2023, Omron, an electrical equipment company based in Japan, announced its plans to open its first medical device plant in India. The new manufacturing plant would be established in the state of Tamil Nadu and is scheduled to commence operations by March 2025. This announcement was made after Tamil Nadu signed the Memoranda of Understanding (MoU) with 6 Japanese companies for a total value of US$ 9.89 million. Omron would invest nearly US$ 15.7 million to set up the plant to manufacture blood pressure monitors.
- In December 2022, Medtronic partnered with BioIntelliSense, a firm offering continuous health monitoring solutions and clinical health intelligence. As part of this partnership, Medtronic would exclusively distribute the BioButton multi-parameter continuous, connected wearable monitoring device in the US. BioButton is rechargeable, and has configurable acute and post-acute modes as patients transition through acuity settings. BioButton records up to 1,440 vital sign measurements daily, including skin temperature, respiratory rate at rest, and rest heart rate. Adding BioButton to Medtronic’s HealthCast connectivity gateway is likely to benefit more general care patients, both inside and outside hospitals, with near real-time measurements and alerts.
The regional trends and factors influencing the Vital Signs Monitoring Devices Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Vital Signs Monitoring Devices Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Vital Signs Monitoring Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 25.62 Billion |
| Market Size by 2030 | US$ 66.82 Billion |
| Global CAGR (2022 - 2030) | 12.7% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Vital Signs Monitoring Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Vital Signs Monitoring Devices Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Vital Signs Monitoring Devices Market top key players overview
Competitive Landscape and Key Companies:
A few prominent players operating in the vital signs monitoring devices market are Koninklijke Philips NV, Medtronic Plc, Nihon Kohden Corp, GE HealthCare Technologies Inc, OMRON Corp, Nonin Medical Inc, SunTech Medical Inc, Masimo Corp, Contec Medical Systems Co Ltd, and Baxter International Inc. These companies focus on new product launches and geographic expansions to meet the growing consumer demand worldwide and increase their product range in specialty portfolios. Their global presence allows them to serve a large set of customers, subsequently allowing them to expand their market share.
Frequently Asked Questions
Based on end user, the market is classified into hospitals & clinics, ambulatory surgical centers, home healthcare, and others.
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For