Brain Cancer Diagnostics Market Insights & Forecast 2034
Brain Cancer Diagnostics Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Diagnostic Type (Imaging Test, Lumbar Puncture, Biopsy, Molecular Testing, Platform and Services, Cerebral Arteriogram, Neurological & Hearing Tests/Neurocognitive Assessments, Electroencephalography ); Cancer Type (Acoustic Neuroma, Astrocytomas, Craniopharyngiomas, Ganglioneuromas, Glioblastoma Multiforme, Meningiomas, Ependymomas, Oligodendroglioma, Low-Grade Tumors ); End User (Hospitals, Specialty Clinics, Diagnostic Centers & Research Institutes, Ambulatory Surgical Centers (ASCs)); Tumor Size, 0.2 cm3 to 100 cm3, 101 cm3 to 200 cm3, Above 200 cm3); and Geography
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Mar 2026
- Report Code : TIPRE00024461
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
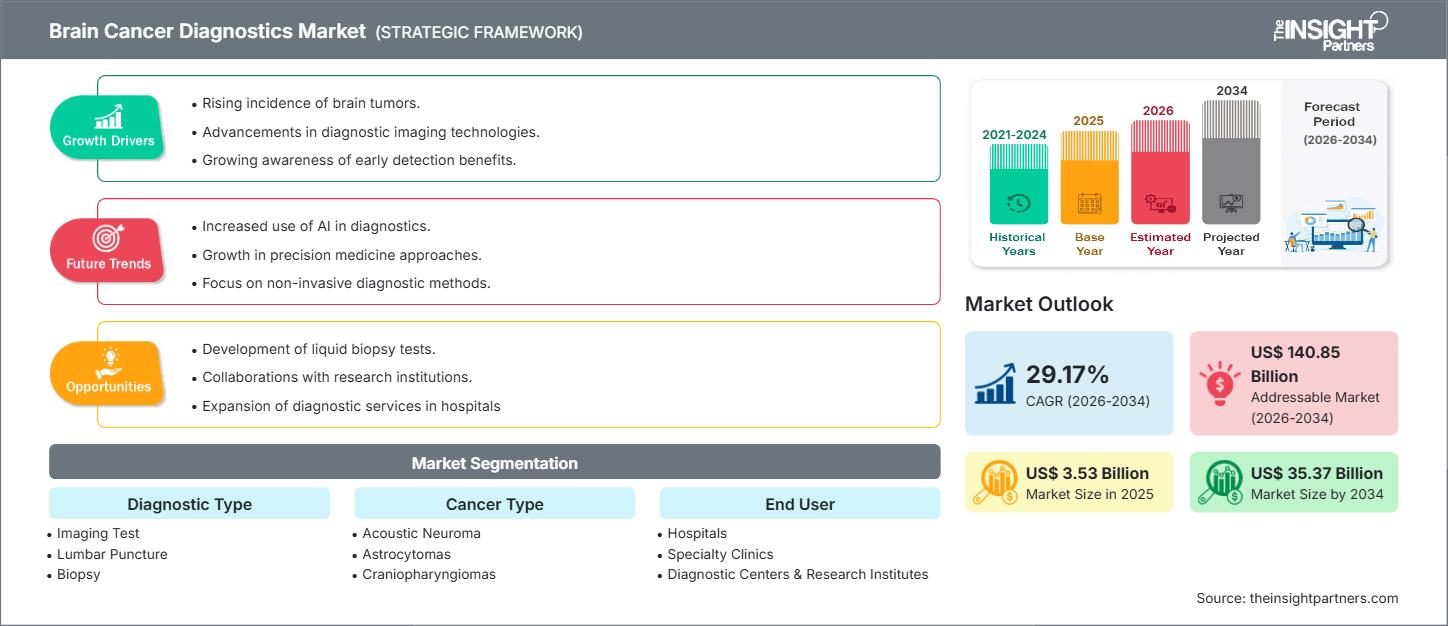
The Brain Cancer Diagnostics Market size is expected to reach US$ 35.37 Billion by 2034 from US$ 3.53 Billion in 2025. The market is anticipated to register a CAGR of 29.17% during 2026–2034.
Brain Cancer Diagnostics Market Analysis
The brain cancer diagnostics market forecast indicates extraordinary growth, driven by the revolutionary integration of advanced technology into neuro-oncology workflows. The acceleration is largely attributed to AI-enabled neuroimaging, which provides quantitative analysis for small-lesion detectability and standardized reporting across various sites. This is supplemented by the rapid adoption of precision molecular profiling, including DNA methylation analysis, Next-Generation Sequencing (NGS) panels, and the emerging field of liquid biopsy from Cerebrospinal Fluid (CSF) and plasma. These cutting-edge tools, exemplified by products like GE HealthCare’s Q.Clear PET quantitation and Philips’ NeuroQuant® integration, allow for highly accurate, reproducible diagnosis and facilitate therapy selection aligned with the latest WHO 2021 Central Nervous System (CNS) classification standards. The market expansion is characterized by the imperative to integrate this complex imaging and genomic data, allowing hospitals and speciality centres to make faster, more confident diagnostic decisions.
Brain Cancer Diagnostics Market Overview
Brain cancer diagnostics comprises a critical suite of sophisticated modalities, including high-field magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET/CT) with perfusion and spectroscopy capabilities, alongside tissue biopsy and less-invasive methods like lumbar puncture. Beyond imaging, the core of the market is shifting towards complex molecular testing, including NGS, methylation profiling, and molecular status assessment (e.g., IDH, MGMT), which are essential for tumor classification and prognosis. Solutions span the entire patient journey, from initial noninvasive screening and precise surgical planning to treatment selection and post-therapy surveillance for both primary tumors (like glioblastoma and meningioma) and metastatic CNS disease. The market's competitive dynamic is defined by rapid AI tool adoption in image interpretation, increasing demand for quantitative, longitudinal analysis, and the expanding clinical validation and reimbursement for multi-analyte liquid biopsy assays.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONBrain Cancer Diagnostics Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Brain Cancer Diagnostics Market Drivers and Opportunities
Market Drivers:
- Rising Incidence of CNS Tumors: The growing global burden of primary and secondary brain cancers across both adult and pediatric populations drives an urgent demand for improved diagnostic sensitivity and specificity, particularly for detecting smaller lesions and providing differential diagnosis early in the disease course.
- AI-Enabled, Quantitative Neuroimaging Standardizing Diagnosis: The integration of deep-learning algorithms into advanced MRI and PET/CT systems (e.g., Siemens Healthineers' AI-Rad Companion) is dramatically improving the quality and reproducibility of imaging. These AI tools enhance small-lesion detectability, provide standardized volumetric analysis, and automate reporting, which is crucial for multidisciplinary team consensus.
- Molecular/Epigenetic Diagnostics Embedded in Clinical Guidelines: The mandatory requirement for molecular data, such as DNA methylation status and specific genetic mutations (IDH, TERT), to classify CNS tumors according to the WHO 2021 guidelines, has firmly established advanced molecular testing as a non-negotiable component of the diagnostic workflow, directly driving the market.
Market Opportunities:
- Clinical Validation and Reimbursement Expansion for Liquid Biopsy: Achieving broader clinical validation and obtaining national reimbursement coverage for highly accurate CSF multi-analyte assays and plasma cfDNA tests represents a huge opportunity to transition from tissue-only diagnostics to routine noninvasive screening and post-treatment recurrence monitoring.
- Companion Diagnostics and Multi-Omics Panels for Precision Therapy: Developing specialized companion diagnostics that predict patient response to targeted therapies (e.g., MGMT promoter methylation testing for temozolomide) and creating multi-omics panels that link tumor genomics, epigenomics, and proteomics will enable hyper-personalized treatment stratification.
- Workflow Automation to Address Specialist Shortages: Implementing AI-driven solutions that automate segmentation, quantitative analysis, and reporting can alleviate the workload on neuroradiologists and pathologists, ensuring that advanced quantitative neuroimaging practices are standardized and scalable across regions facing specialist shortages.
Brain Cancer Diagnostics Market Report Segmentation Analysis
The brain cancer diagnostics market share is analyzed across various segments to provide a clearer understanding of its structure, growth potential, and emerging technological trends. Below is a detailed segmentation approach used in most industry reports:
By Diagnostic Type:
- Imaging Test
- Lumbar Puncture
- Biopsy
- Molecular Testing
- Platform and Services
- Cerebral Arteriogram
- Neurological & Hearing Tests/Neurocognitive Assessments
- Electroencephalography
By Cancer Type:
- Acoustic Neuroma
- Astrocytomas
- Craniopharyngiomas
- Ganglioneuromas
- Glioblastoma Multiforme
- Meningiomas
- Ependymomas
- Oligodendroglioma
- Low-Grade Tumors
By End User:
- Hospitals
- Specialty Clinics
- Diagnostic Centers & Research Institutes
- Ambulatory Surgical Centers (ASCs)
By Tumor Size
- 0.2 cm3 to 100 cm3
- 101 cm3 to 200 cm3
- Above 200 cm3
By Geography:
- North America
- Europe
- Asia Pacific
- South & Central America
- Middle East & Africa
The regional trends and factors influencing the Brain Cancer Diagnostics Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Brain Cancer Diagnostics Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Brain Cancer Diagnostics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 3.53 Billion |
| Market Size by 2034 | US$ 35.37 Billion |
| Global CAGR (2026 - 2034) | 29.17% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Diagnostic Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Brain Cancer Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Brain Cancer Diagnostics Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Brain Cancer Diagnostics Market top key players overview
Brain Cancer Diagnostics Market Share Analysis by Geography
The brain cancer diagnostics market exhibits distinct growth and technology adoption patterns across global regions, influenced by healthcare spending, regulatory alignment with precision medicine, and the existing infrastructure for advanced diagnostics. Below is a summary of market share and trends by region:
North America
- Market Share: Holds the largest market share globally due to mature regulatory pathways, high healthcare expenditure, and a strong culture of adopting cutting-edge technologies.
- Key Drivers: Favorable reimbursement environment for advanced genomic and epigenetic testing.
- Trends: Strong shift towards multi-omics data integration and the development of vendor-neutral platforms that connect imaging, pathology, and genomics data.
Europe
- Market Share: Significant market share, driven by strong academic research in neuro-oncology and increasing national healthcare system commitment to precision medicine.
- Key Drivers: High demand for harmonizing diagnostic standards across different countries.
- Trends: Focus on implementing standardized quantitative neuroimaging protocols and expanding access to molecular testing through centralized reference laboratories.
Asia Pacific
- Market Share: Fastest-growing region, driven by massive investments in oncology infrastructure and expanding patient populations.
- Key Drivers: Increasing adoption of NGS panels to address the rising brain cancer burden.
- Trends: High demand for cost-effective, scalable diagnostic solutions; growing interest in local clinical validation of advanced liquid biopsy techniques.
South and Central America
- Market Share: Emerging market with developing digital health and specialized diagnostics infrastructure.
- Key Drivers: Expansion of private oncology clinics leading the adoption of scalable, newer technologies.
- Trends: Cloud-based imaging reading and tele-radiology solutions are gaining traction to overcome geographic barriers and specialist shortages.
Middle East and Africa
- Market Share: Developing market with strong growth potential fueled by national healthcare modernization initiatives.
- Key Drivers: Increasing access to advanced imaging equipment in major cities.
- Trends: Focus on establishing foundational diagnostic capabilities and gradually integrating AI tools to maximize the efficiency of newly acquired imaging equipment.
Brain Cancer Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition in the brain cancer diagnostics market is intense and fragmented, spanning two distinct technology areas: advanced imaging and precision molecular diagnostics. Major vendors such as Siemens Healthineers AG, GE HealthCare, and Koninklijke Philips N.V. dominate the imaging space, while companies like THERMO FISHER SCIENTIFIC INC and MDxHealth lead the molecular and genomic segments. Regional and niche molecular players like Biocept, Inc. also contribute significantly to the competitive landscape.
This environment pushes vendors to differentiate through:
- AI Integration: Embedding AI algorithms directly into imaging hardware and software to automate quantitative measurements (e.g., tumor volume) and standardize reporting.
- Multi-Modal Platforms: Offering solutions that can seamlessly combine data from imaging, pathology, and genomics to create a single, comprehensive patient profile.
- Minimally Invasive Diagnostics: Investing heavily in validating and commercializing noninvasive liquid biopsy assays (CSF and plasma) to reduce patient risk and improve monitoring frequency.
- Alignment with Guidelines: Ensuring that molecular diagnostic offerings are perfectly aligned with the latest clinical classification standards, such as the WHO 2021 CNS criteria.
Major Companies operating in the Brain Cancer Diagnostics Market are:
- THERMO FISHER SCIENTIFIC INC
- Siemens Healthineers AG
- GE HealthCare
- Koninklijke Philips N.V.
- MDxHealth
- Biocept, Inc.
- Canon Medical Systems
- Hitachi, Ltd
- NantOmics
Disclaimer: The companies listed above are not ranked in any particular order.
Brain Cancer Diagnostics Market News and Recent Developments
- Thermo Fisher Scientific Inc. is advancing brain tumor biomarker testing through its Oncomine™ NGS solutions, including the Oncomine Dx Express Test powered by the Ion Torrent Genexus Dx system. The company also supports spatial omics in neuroscience, enabling dual ISH–IHC brain mapping, which preserves deep molecular context to enhance brain cancer diagnostics research.
- Siemens Healthineers AG continues to strengthen neuroradiology and neuro-oncology capabilities through its Neurology Biomarkers and Assays, including blood-based neurofilament light chain testing. Siemens’ innovations in imaging, such as MAGNETOM and Biograph mMR systems, Deep Resolve AI reconstruction, and the photon-counting CT NAEOTOM Alpha, offer improved tumor imaging precision and diagnostic reliability.
Brain Cancer Diagnostics Market Report Coverage and Deliverables
The "Brain Cancer Diagnostics Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering below areas:
- Brain Cancer Diagnostics Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Brain Cancer Diagnostics Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Brain Cancer Diagnostics Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the Brain Cancer Diagnostics Market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For