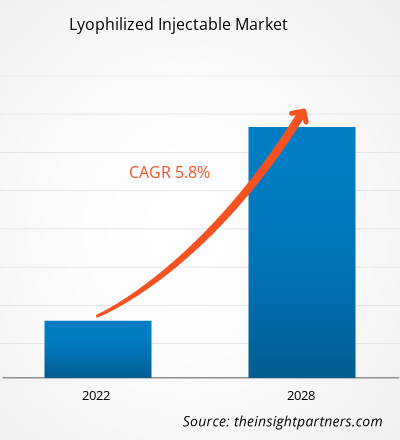
Se proyecta que el mercado de inyectables liofilizados alcance los US$ 4.001,27 millones para 2028 desde los US$ 2.719,42 millones en 2021; se espera que crezca a una CAGR del 5,8% entre 2022 y 2028.
Los factores clave que impulsan el mercado son la creciente demanda de servicios de fabricación para investigación por contrato, el aumento de las aprobaciones de productos farmacéuticos y la creciente demanda de productos biológicos. Sin embargo, las retiradas de productos frenan el crecimiento del mercado.
Los inyectables liofilizados son medicamentos liofilizados que se mantienen estables durante el transporte. Se almacenan en viales de un solo uso, en envases especiales para la reconstitución en el punto de atención y durante un período más prolongado. Al momento de su uso, se puede reconstituir la cantidad necesaria de medicamento con la ayuda de diluyentes. Los inyectables liofilizados también se denominan inyectables liofilizados, ya que se fabrican mediante el proceso de liofilización, una técnica que consiste en aislar un material sólido de un disolvente mediante la congelación y evaporación de la solución al vacío. Las inyecciones liofilizadas suelen considerarse la mejor alternativa a las formas farmacéuticas sólidas orales. En muchos estudios de caso, las inyecciones han beneficiado enormemente a los pacientes encamados. Las inyecciones liofilizadas también se prescriben para lograr la máxima biodisponibilidad y estabilidad en pacientes que padecen diversas enfermedades, ya que estos inyectables tienen una vida útil más larga que otras formas farmacéuticas.
Obtendrá personalización en cualquier informe, sin cargo, incluidas partes de este informe o análisis a nivel de país, paquete de datos de Excel, así como también grandes ofertas y descuentos para empresas emergentes y universidades.
Mercado de inyectables liofilizados: Perspectivas estratégicas

-
Obtenga las principales tendencias clave del mercado de este informe.Esta muestra GRATUITA incluirá análisis de datos, desde tendencias del mercado hasta estimaciones y pronósticos.
Perspectivas del mercado
Las crecientes aprobaciones de productos farmacéuticos impulsan el crecimiento del mercado
Se están realizando estudios sobre la seguridad y eficacia de nuevos agentes terapéuticos liofilizados utilizados en pacientes adultos hospitalizados con diagnóstico de COVID-19. Los fabricantes del mercado global de liofilizados inyectables están solicitando la aprobación de la FDA estadounidense para la autorización de uso de emergencia (EUA) de estas innovaciones para tratar a pacientes con síntomas graves de COVID-19.
Desde la pandemia de COVID-19, los actores del mercado global de inyectables liofilizados están intensificando sus esfuerzos para obtener la aprobación de organismos reguladores, como el Controlador General de Medicamentos de la India (DCGI), para su uso restringido de emergencia en India. El aumento de las aprobaciones de fármacos, como el Remdesivir, ha propiciado una alta demanda de inyectables liofilizados. A continuación, se presentan algunos ejemplos de fármacos aprobados por la FDA y otros organismos reguladores.
- En febrero de 2020, Mylan obtuvo la aprobación regulatoria de DCGI para el polvo liofilizado de Remdesivir en India para uso de emergencia restringido en pacientes con COVID-19.
- En junio de 2020, Cipla lanzó Cipremi, Remdesivir en polvo liofilizado inyectable. Además, la FDA de EE. UU. emitió una Autorización de Uso de Emergencia (EUA) a Gilead Sciences Inc. para el uso de emergencia de Remdesivir en el tratamiento de pacientes hospitalizados con COVID-19. Es el único tratamiento con EUA aprobado por la FDA para pacientes adultos y pediátricos hospitalizados con infección por COVID-19 presunta o confirmada por laboratorio. Por lo tanto, en mayo, Gilead Sciences Inc. extendió una licencia voluntaria no exclusiva a Cipla para fabricar y comercializar su versión genérica de remedisvir, denominada CIPREMI.
- En julio de 2020, Jubilant Life recibió la aprobación del Controlador General de Medicamentos de la India (DCGI) para fabricar y comercializar Remdesivir en dosis de 100 mg/vial (inyección liofilizada) para uso de emergencia restringido en la India para el tratamiento de COVID-19 grave.
- En agosto de 2020, Cosentyx, polvo liofilizado en vial de un solo uso de Novartis AG, recibió la aprobación de la UE. El fármaco aprobado demostró ser muy eficaz para mejorar rápidamente los síntomas cutáneos y la calidad de vida de los pacientes con psoriasis.
Así, las crecientes aprobaciones en todo el mundo impulsan el crecimiento del mercado de inyectables liofilizados.lyophilized injectables market.
Tipo de embalaje: información basada en datos
El mercado global de inyectables liofilizados, según el tipo de envase, se segmenta en viales de un solo uso, reconstitución en el punto de atención y envases especiales. En 2021, el segmento de viales de un solo uso representó la mayor cuota de mercado, mientras que se espera que el segmento de envases especiales registre la mayor tasa de crecimiento anual compuesta (TCAC) del 6,7 % durante el período de pronóstico.
Tipo de información basada en la entrega
Según el tipo de administración, el mercado global de inyectables liofilizados se segmenta en jeringas precargadas con diluyente, dispositivos de reconstitución patentados, dispositivos de un solo paso y dispositivos de varios pasos. El segmento de jeringas precargadas con diluyente tuvo la mayor participación de mercado en 2021 y se prevé que registre la mayor tasa de crecimiento anual compuesta (TCAC) del 6,3 % durante el período de pronóstico.
Perspectivas basadas en indicaciones
Según las indicaciones, el mercado global de inyectables liofilizados se segmenta en enfermedades metabólicas y oncológicas, enfermedades infecciosas, enfermedades autoinmunes y otras indicaciones. Este segmento tuvo la mayor participación de mercado en 2021 y se prevé que registre la mayor tasa de crecimiento anual compuesta (TCAC) del 6,2 % durante el período de pronóstico.
Información basada en el usuario final
Según el usuario final, el mercado global de inyectables liofilizados se segmenta en hospitales, centros de cirugía ambulatoria, clínicas especializadas y otros. El segmento de hospitales tuvo la mayor participación de mercado en 2021. Sin embargo, se estima que el segmento de clínicas especializadas registrará la tasa de crecimiento anual compuesta (TCAC) más alta del mercado, del 6,3 % durante el período de pronóstico.
Las empresas que operan en el mercado global de inyectables liofilizados adoptan una estrategia de innovación de productos para satisfacer la creciente demanda de los clientes a nivel mundial, lo que también les permite mantener su marca en el mercado global. Por ejemplo, en abril de 2022, Recipharm adquirió Arranta Bio, empresa de desarrollo de marca de terapia avanzada, y Vibalogics, empresa de desarrollo de marca de viroterapia. Esta adquisición también permitió a Recipharm establecer una sólida base en EE. UU., con instalaciones en Boxborough, Massachusetts, y proporcionar una plataforma desde la cual desarrollar sus capacidades en nuevas modalidades de productos biológicos.
Perspectivas regionales del mercado de inyectables liofilizados Injectable
Los analistas de The Insight Partners han explicado detalladamente las tendencias regionales y los factores que influyen en el mercado de inyectables liofilizados durante el período de pronóstico. Esta sección también analiza los segmentos y la geografía del mercado de inyectables liofilizados en América del Norte, Europa, Asia Pacífico, Oriente Medio y África, y América del Sur y Central.
Alcance del informe de mercado de inyectables liofilizados
| Atributo del informe | Detalles |
|---|---|
| Tamaño del mercado en 2021 | US$ 2.72 mil millones |
| Tamaño del mercado en 2028 | 4 mil millones de dólares estadounidenses |
| CAGR global (2021-2028) | 5,8% |
| Datos históricos | 2019-2020 |
| Período de pronóstico | 2022-2028 |
| Segmentos cubiertos |
Por tipo de embalaje
|
| Regiones y países cubiertos |
América del norte
|
| Líderes del mercado y perfiles de empresas clave |
|
Densidad de actores del mercado de inyectables liofilizados: comprensión de su impacto en la dinámica empresarial
El mercado de inyectables liofilizados está creciendo rápidamente, impulsado por la creciente demanda del usuario final debido a factores como la evolución de las preferencias del consumidor, los avances tecnológicos y un mayor conocimiento de los beneficios del producto. A medida que aumenta la demanda, las empresas amplían su oferta, innovan para satisfacer las necesidades del consumidor y aprovechan las tendencias emergentes, lo que impulsa aún más el crecimiento del mercado.
- Obtenga una descripción general de los principales actores clave del mercado de inyectables liofilizados
Mercado global de liofilizados inyectables por geografía
Geográficamente, el mercado global de inyectables liofilizados está segmentado en América del Norte (EE. UU., Canadá y México), Europa (Alemania, Reino Unido, Francia, Italia, España y el resto de Europa), Asia Pacífico (China, India, Japón, Corea del Sur, Australia y el resto de APAC), Medio Oriente y África (Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos y el resto de MEA) y América del Sur y Central (Brasil, Argentina y el resto de América del Sur y Central).
Perfiles de empresas
- Baxter
- Nipro
- Curia Global, Inc.
- Recipharm AB
- Vetter Pharma
- Jubilant HollisterStier LLC (Jubilant Pharma Limited)
- Aristopharma Ltd.
- CordenPharma Internacional
- Credence MedSystems, Inc.
- SG Biopharm Pvt. Ltd
- Análisis histórico (2 años), año base, pronóstico (7 años) con CAGR
- Análisis PEST y FODA
- Tamaño del mercado, valor/volumen: global, regional y nacional
- Industria y panorama competitivo
- Conjunto de datos de Excel
Informes recientes
Testimonios
Razón para comprar
- Toma de decisiones informada
- Comprensión de la dinámica del mercado
- Análisis competitivo
- Información sobre clientes
- Pronósticos del mercado
- Mitigación de riesgos
- Planificación estratégica
- Justificación de la inversión
- Identificación de mercados emergentes
- Mejora de las estrategias de marketing
- Impulso de la eficiencia operativa
- Alineación con las tendencias regulatorias






















 Obtenga una muestra gratuita para - Mercado de inyectables liofilizados
Obtenga una muestra gratuita para - Mercado de inyectables liofilizados