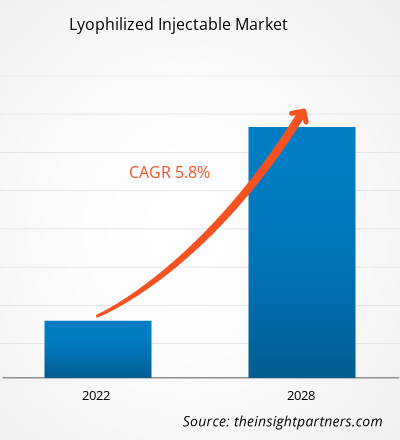
凍結乾燥注射剤市場は、2021年の27億1,942万米ドルから2028年には40億127万米ドルに達すると予測されており、2022年から2028年にかけて5.8%のCAGRで成長すると見込まれています。
市場を牽引する主な要因は、受託研究製造サービスの需要増加、医薬品の承認件数の増加、および生物製剤の需要増加です。しかし、製品リコールが市場の成長を抑制しています。
凍結乾燥注射剤は、輸送中に安定した凍結乾燥薬剤です。使い捨てバイアル、ポイントオブケア再構成、および特殊包装で長期間保管されます。使用時には、希釈剤を使用して必要量の薬剤を再構成できます。凍結乾燥注射剤は、凍結乾燥法(溶液を真空下で凍結・蒸発させることで固体物質を溶媒から分離する技術)によって製造されるため、フリーズドライ注射剤とも呼ばれます。凍結乾燥注射剤は、経口固形剤の最良の代替品とみなされることが多く、多くの症例研究において、寝たきりの患者に多大な効果が認められています。また、凍結乾燥注射剤は他の剤形よりも保存期間が長いため、様々な疾患の患者において最大限のバイオアベイラビリティと安定性を得るために処方されています。
要件に合わせてレポートをカスタマイズ
レポートの一部、国レベルの分析、Excelデータパックなどを含め、スタートアップ&大学向けに特別オファーや割引もご利用いただけます(無償)
凍結乾燥注射剤市場: 戦略的洞察

-
このレポートの主要な市場動向を入手してください。この無料サンプルには、市場動向から見積もりや予測に至るまでのデータ分析が含まれます。
市場分析
医薬品の承認増加が市場の成長を牽引
COVID-19と診断された入院中の成人患者に使用される新しい凍結乾燥治療薬の安全性と有効性に関する研究が進行中です。世界の凍結乾燥注射剤市場のメーカーは、重度のCOVID-19症状に苦しむ患者を治療するために、米国FDAからこのような革新的な薬剤の緊急使用許可(EUA)の承認を取得しています。
COVID-19パンデミック以降、世界の凍結乾燥注射剤市場のプレーヤーは、インドでの限定的な緊急使用について、インド医薬品管理総局(DCGI)などの規制機関の承認を得るための取り組みを強化しています。レムデシビルなどの医薬品の承認増加は、最終的に凍結乾燥注射剤の需要の高まりにつながります。以下は、FDAやその他の規制当局によって承認された医薬品の例です。
- 2020年2月、マイラン社はインドにおいて、COVID-19患者への限定的な緊急使用を目的として、レムデシビル凍結乾燥粉末の規制承認をDCGIから取得しました。
- 2020年6月、シプラ社は注射用レムデシビル凍結乾燥粉末「シプレミ」を発売しました。また、米国FDAはギリアド・サイエンシズ社に対し、COVID-19の入院患者の治療を目的としたレムデシビルの緊急使用許可(EUA)を発行しました。これは、COVID-19感染の疑いがある、または検査で感染が確認された入院中の成人および小児患者に対する、FDA承認の唯一のEUA治療薬です。そのため、5月にギリアド・サイエンシズ社は、シプラ社に対し、シプラ社のレメディスビルのジェネリック版であるCIPREMIの製造および販売を行うための非独占的自主ライセンスを延長しました。
- 2020年7月、ジュビラント・ライフ社は、インド医薬品管理総局(DCGI)から、重症COVID-19の治療のため、インドでの限定的な緊急使用のために、レムデシビル100mg/バイアル(凍結乾燥注射剤)の製造および販売の承認を取得しました。
- 2020年8月、ノバルティス社製の使い捨てバイアルに入ったコセンティクス凍結乾燥粉末がEUの承認を取得しました。承認された医薬品は、皮膚症状を急速に改善し、乾癬に苦しむ患者の生活の質を向上させるのに非常に効果的でした。
このように、世界中で承認が増えていることが、凍結乾燥注射剤市場の成長を促進しています。
パッケージの種類に基づく洞察
世界の凍結乾燥注射剤市場は、パッケージの種類に基づいて、使い捨てバイアル、ポイントオブケア再構成、および特殊パッケージに分類されています。 2021年には、使い捨てバイアルセグメントが最大の市場シェアを占めましたが、予測期間中に特殊包装セグメントが6.7%という最高のCAGRを記録すると予想されています。
送達タイプに基づく洞察
送達タイプに基づいて、世界の凍結乾燥注射剤市場は、充填済み希釈剤シリンジ、独自の再構成デバイス、シングルステップデバイス、およびマルチステップデバイスに分類されています。充填済み希釈剤シリンジセグメントは2021年に最大の市場シェアを占め、予測期間中に6.3%という最高のCAGRを記録すると予想されています。
適応症に基づく洞察
適応症に基づいて、世界の凍結乾燥注射剤市場は、代謝および腫瘍学的状態、感染症、自己免疫疾患、およびその他の適応症に分類されています。代謝および腫瘍学的疾患セグメントは、2021年に最大の市場シェアを占め、予測期間中に6.2%という最高のCAGRを記録すると予想されています。
エンドユーザーベースの洞察
エンドユーザーに基づいて、世界の凍結乾燥注射剤市場は、病院、外来手術センター、専門クリニック、その他に分類されています。病院セグメントは2021年に最大の市場シェアを占めました。しかし、専門クリニックセグメントは、予測期間中に市場で6.3%という最高のCAGRを記録すると予測されています。
世界の凍結乾燥注射剤市場で事業を展開している企業は、世界中で進化する顧客の需要を満たすために製品イノベーション戦略を採用しており、これにより、世界市場でブランド名を維持することもできます。たとえば、2022年4月、Recipharmは先進療法CDMO Arranta Bioとウイルス療法CDMO Vibalogicsを買収しました。また、この買収により、レシファームはマサチューセッツ州ボックスボロに施設を構え、米国に強固な基盤を確立し、新たな生物製剤のモダリティにおける能力を構築するためのプラットフォームを提供することができました。
凍結乾燥注射剤市場の地域別分析
The Insight Partnersのアナリストは、予測期間全体を通して凍結乾燥注射剤市場に影響を与える地域的な動向と要因を詳細に解説しています。このセクションでは、北米、ヨーロッパ、アジア太平洋、中東・アフリカ、中南米における凍結乾燥注射剤市場のセグメントと地域についても解説しています。
凍結乾燥注射剤市場レポートの範囲
| レポート属性 | 詳細 |
|---|---|
| の市場規模 2021 | US$ 2.72 Billion |
| 市場規模別 2028 | US$ 4 Billion |
| 世界的なCAGR (2021 - 2028) | 5.8% |
| 過去データ | 2019-2020 |
| 予測期間 | 2022-2028 |
| 対象セグメント |
By 包装の種類
|
| 対象地域と国 |
北米
|
| 市場リーダーと主要企業の概要 |
|
凍結乾燥注射剤市場のプレーヤー密度:ビジネスダイナミクスへの影響を理解する
凍結乾燥注射剤市場は、消費者の嗜好の変化、技術の進歩、製品の利点に対する認知度の高まりといった要因によるエンドユーザーの需要増加に牽引され、急速に成長しています。需要の増加に伴い、企業は製品ラインナップの拡充、消費者ニーズへの対応のための革新、そして新たなトレンドの活用を進めており、これが市場の成長をさらに加速させています。
- 入手 凍結乾燥注射剤市場 主要プレーヤーの概要
世界の凍結乾燥注射剤市場 - 地域別
地理的に見ると、世界の凍結乾燥注射剤市場は、北米 (米国、カナダ、メキシコ)、ヨーロッパ (ドイツ、イギリス、フランス、イタリア、スペイン、その他のヨーロッパ諸国)、アジア太平洋 (中国、インド、日本、韓国、オーストラリア、その他のアジア太平洋諸国)、中東およびアフリカに分割されています。
企業プロファイル
- バクスター
- ニプロ
- キュリア グローバル インク
- レシファーム AB
- ベッター ファーマ
- ジュビラント ホリスター スティアー LLC (ジュビラント ファーマ リミテッド)
- アリストファーマ 株式会社
- コーデンファーマ インターナショナル
- クレデンス メドシステムズ株式会社
- SG バイオファーマ プライベート リミテッド
- 過去2年間の分析、基準年、CAGRによる予測(7年間)
- PEST分析とSWOT分析
- 市場規模価値/数量 - 世界、地域、国
- 業界と競争環境
- Excel データセット
最新レポート
お客様の声
購入理由
- 情報に基づいた意思決定
- 市場動向の理解
- 競合分析
- 顧客インサイト
- 市場予測
- リスク軽減
- 戦略計画
- 投資の正当性
- 新興市場の特定
- マーケティング戦略の強化
- 業務効率の向上
- 規制動向への対応






















 無料サンプルを入手 - 凍結乾燥注射剤市場
無料サンプルを入手 - 凍結乾燥注射剤市場