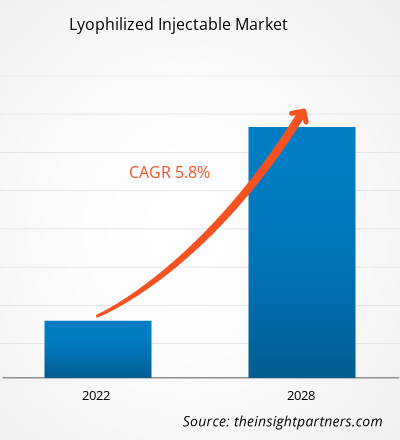
Le marché des injectables lyophilisés devrait atteindre 4 001,27 millions de dollars américains d'ici 2028, contre 2 719,42 millions de dollars américains en 2021 ; il devrait croître à un TCAC de 5,8 % entre 2022 et 2028.
Les principaux facteurs qui stimulent le marché sont la demande croissante de services de fabrication de recherche sous contrat, l'augmentation des approbations de produits pharmaceutiques et la demande croissante de produits biologiques. Cependant, les rappels de produits freinent la croissance du marché.
Les injectables lyophilisés sont des médicaments lyophilisés qui sont stables pendant le transport. Ils sont stockés dans des flacons à usage unique, reconstitués au point de service et conditionnés dans des emballages spéciaux pendant une période plus longue. Au moment de l'utilisation, la quantité requise de médicaments peut être reconstituée à l'aide de diluants. Les injectables lyophilisés sont également appelés injectables lyophilisés car ils sont fabriqués par lyophilisation, une technique qui consiste à isoler un solide d'un solvant par congélation et évaporation de la solution sous vide. Les injections lyophilisées sont souvent considérées comme la meilleure alternative aux formes posologiques solides orales. Dans de nombreuses études de cas, ces injections ont été extrêmement bénéfiques pour les patients alités. Les injections lyophilisées sont également prescrites pour optimiser la biodisponibilité et la stabilité chez les patients souffrant de diverses maladies, car ces injectables ont une durée de conservation plus longue que les autres formes posologiques.
Vous bénéficierez d’une personnalisation sur n’importe quel rapport - gratuitement - y compris des parties de ce rapport, ou une analyse au niveau du pays, un pack de données Excel, ainsi que de profiter d’offres exceptionnelles et de réductions pour les start-ups et les universités
Marché des injectables lyophilisés: Perspectives stratégiques

-
Obtenez les principales tendances clés du marché de ce rapport.Cet échantillon GRATUIT comprendra une analyse de données, allant des tendances du marché aux estimations et prévisions.
Aperçu du marché
L'augmentation des approbations de produits pharmaceutiques stimule la croissance du marché
Des études sont en cours sur la sécurité et l'efficacité de nouveaux agents thérapeutiques lyophilisés utilisés chez les patients adultes hospitalisés diagnostiqués avec la COVID-19. Les fabricants du marché mondial des injectables lyophilisés attendent l'approbation de la FDA américaine pour l'autorisation d'utilisation d'urgence (EUA) de ces innovations afin de traiter les patients souffrant de symptômes sévères de la COVID-19.
Depuis la pandémie de COVID-19, les acteurs du marché mondial des injectables lyophilisés redoublent d'efforts pour obtenir l'approbation des organismes de réglementation, tels que le Drug Controller General of India (DCGI), pour une utilisation d'urgence restreinte en Inde. L'augmentation des approbations de produits pharmaceutiques, comme le Remdesivir, conduit finalement à une forte demande d'injectables lyophilisés. Voici quelques exemples de produits pharmaceutiques approuvés par la FDA et d'autres organismes de réglementation.
- En février 2020, Mylan a obtenu l'approbation réglementaire du DCGI pour la poudre lyophilisée de Remdesivir en Inde pour une utilisation d'urgence restreinte chez les patients atteints de la COVID-19.
- En juin 2020, Cipla a lancé Cipremi, la poudre lyophilisée de Remdesivir pour injection. De plus, la FDA américaine a délivré une autorisation d'utilisation d'urgence (EUA) à Gilead Sciences Inc. pour l'utilisation d'urgence du Remdesivir afin de traiter les patients hospitalisés atteints de la COVID-19. Il s'agit du seul traitement EUA approuvé par la FDA pour les patients adultes et pédiatriques hospitalisés avec une infection à la COVID-19 suspectée ou confirmée en laboratoire. Ainsi, en mai, Gilead Sciences Inc. a accordé une licence volontaire non exclusive à Cipla pour fabriquer et commercialiser la version générique du remedisvir de Cipla appelée CIPREMI.
- En juillet 2020, Jubilant Life a reçu l'approbation du Drug Controller General of India (DCGI) pour fabriquer et commercialiser du Remdesivir à 100 mg/flacon (injection lyophilisée) pour une utilisation d'urgence restreinte en Inde pour le traitement des formes graves de COVID-19.
- En août 2020, la poudre lyophilisée Cosentyx dans un flacon à usage unique de Novartis AG a reçu l'approbation de l'UE. Le médicament approuvé s'est avéré très productif en améliorant rapidement les symptômes cutanés et en améliorant la qualité de vie des patients souffrant de psoriasis.
Ainsi, les approbations croissantes à travers le monde propulsent la croissance du marché des injectables lyophilisés.
Informations basées sur le type d'emballage
Le marché mondial des injectables lyophilisés, en fonction du type d'emballage, est segmenté en flacons à usage unique, reconstitution au point de service et emballages spécialisés. Français En 2021, le segment des flacons à usage unique représentait la plus grande part de marché, tandis que le segment des emballages spécialisés devrait enregistrer le TCAC le plus élevé de 6,7 % au cours de la période de prévision.
Informations basées sur le type d'administration
En fonction du type d'administration, le marché mondial des injectables lyophilisés est segmenté en seringues de diluant préremplies, dispositifs de reconstitution exclusifs, dispositifs à une seule étape et dispositifs à plusieurs étapes. Le segment des seringues de diluant préremplies détenait la plus grande part de marché en 2021 et devrait enregistrer le TCAC le plus élevé de 6,3 % au cours de la période de prévision.
Informations basées sur l'indication
En fonction de l'indication, le marché mondial des injectables lyophilisés est segmenté en affections métaboliques et oncologiques, maladies infectieuses, maladies auto-immunes et autres indications. Français Le segment des affections métaboliques et oncologiques détenait la plus grande part de marché en 2021 et devrait enregistrer le TCAC le plus élevé de 6,2 % au cours de la période de prévision.
Informations basées sur l'utilisateur final
Sur la base de l'utilisateur final, le marché mondial des injectables lyophilisés est segmenté en hôpitaux, centres de chirurgie ambulatoire, cliniques spécialisées et autres. Le segment des hôpitaux détenait la plus grande part de marché en 2021. Cependant, le segment des cliniques spécialisées devrait enregistrer le TCAC le plus élevé du marché, soit 6,3 % au cours de la période de prévision.
Les entreprises opérant sur le marché mondial des injectables lyophilisés adoptent une stratégie d'innovation produit pour répondre à l'évolution de la demande des clients à travers le monde, ce qui leur permet également de maintenir leur marque sur le marché mondial. Par exemple, en avril 2022, Recipharm a acquis Arranta Bio, CDMO de thérapie avancée, et Vibalogics, CDMO de virothérapie. L'acquisition a également permis à Recipharm d'établir une base solide aux États-Unis, avec des installations à Boxborough, dans le Massachusetts, et de fournir une plate-forme à partir de laquelle développer ses capacités dans de nouvelles modalités biologiques.
Aperçu régional du marché des injectables lyophilisés
Les tendances régionales et les facteurs influençant le marché des injectables lyophilisés tout au long de la période de prévision ont été analysés en détail par les analystes de The Insight Partners. Cette section aborde également les segments et la géographie du marché des injectables lyophilisés en Amérique du Nord, en Europe, en Asie-Pacifique, au Moyen-Orient et en Afrique, ainsi qu'en Amérique du Sud et en Amérique centrale.
Portée du rapport sur le marché des injections lyophilisées
| Attribut de rapport | Détails |
|---|---|
| Taille du marché en 2021 | US$ 2.72 Billion |
| Taille du marché par 2028 | US$ 4 Billion |
| TCAC mondial (2021 - 2028) | 5.8% |
| Données historiques | 2019-2020 |
| Période de prévision | 2022-2028 |
| Segments couverts |
By Type d'emballage
|
| Régions et pays couverts |
Amérique du Nord
|
| Leaders du marché et profils d'entreprises clés |
|
Densité des acteurs du marché des injectables lyophilisés : comprendre son impact sur la dynamique commerciale
Le marché des injectables lyophilisés connaît une croissance rapide, portée par une demande croissante des utilisateurs finaux, due à des facteurs tels que l'évolution des préférences des consommateurs, les avancées technologiques et une meilleure connaissance des avantages du produit. Face à cette demande croissante, les entreprises élargissent leur offre, innovent pour répondre aux besoins des consommateurs et capitalisent sur les nouvelles tendances, ce qui alimente la croissance du marché.
- Obtenez le Marché des injectables lyophilisés Aperçu des principaux acteurs clés
- Analyse historique (2 ans), année de base, prévision (7 ans) avec TCAC
- Analyse PEST et SWOT
- Taille du marché Valeur / Volume - Mondial, Régional, Pays
- Industrie et paysage concurrentiel
- Ensemble de données Excel
Rapports récents
Témoignages
Raison d'acheter
- Prise de décision éclairée
- Compréhension de la dynamique du marché
- Analyse concurrentielle
- Connaissances clients
- Prévisions de marché
- Atténuation des risques
- Planification stratégique
- Justification des investissements
- Identification des marchés émergents
- Amélioration des stratégies marketing
- Amélioration de l'efficacité opérationnelle
- Alignement sur les tendances réglementaires






















 Obtenez un échantillon gratuit pour - Marché des injectables lyophilisés
Obtenez un échantillon gratuit pour - Marché des injectables lyophilisés