Europe and Middle East Spinal Cord Stimulation Devices for Chronic Pain Market Growth by 2030
Europe and Middle East Spinal Cord Stimulation Devices for Chronic Pain Market Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Product Type (Rechargeable and Non-Rechargeable), Application (Failed Back Syndrome, Complex Regional Pain Syndrome, Degenerative Disk Disease, and Others), End User [Hospitals, Ambulatory Surgery Centers (ASCs), and Others], and Regional Analysis
Historic Data: 2020-2021 | Base Year: 2022 | Forecast Period: 2023-2030- Report Date : Nov 2023
- Report Code : TIPRE00030317
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 204
The spinal cord stimulation devices for chronic pain market size is projected to grow from US$ 892.66 million in 2022 to US$ 1,573.67 million by 2030; the market is estimated to register a CAGR of 7.3% from 2022 to 2030.
Analyst’s View Point
The Europe and Middle East spinal cord stimulation devices for chronic pain market analysis explains driving factors such as the rising number of spinal cord injuries (SCIs) and the availability of innovative treatments in the European Union (EU). Further, regenerative and innovative implant-based therapeutic approaches are expected to introduce new trends into the market during 2022–2030. Based on product type, the rechargeable segment accounted for a larger share of the market in 2022. Based on application, the complex regional pain syndrome segment dominated the Europe and Middle East spinal cord stimulation devices for chronic pain market by accounting for a maximum share in 2022. In terms of end user, the hospitals segment is likely to hold a considerable share of the market in Europe and Middle East during the forecast period.
A spinal cord stimulation device is an implanted device that delivers pain relief by transmitting low-level electrical signals directly into the spinal cord. The stimulator is mostly used after nonsurgical pain treatments fail to provide sufficient relief. Additionally, the device can improve the overall quality of life and sleep. As it is typically prescribed along with other pain management treatments, it effectively suppresses the need for pain medicines.
Market Insights
Rising Number of Spinal Cord Injuries Bolsters Europe and Middle East Spinal Cord Stimulation Devices for Chronic Pain Market
According to a report published by the Association for Spinal Injury Research, Rehabilitation and Reintegration (Aspire, UK), ~2,500 people are diagnosed with spinal cord injuries (SCIs) annually in the UK. Currently, ~50,000 people are living with SCIs in the country. An elevated number of cases is attributed to emerging motocross and motorcycling competitions as the most popular sporting activities in the UK, as per the National Institute of Health (NIH) report. With rising motor sporting activities among the youth and senior age group population, there is an increase in the incidence of SCIs among the participants of these events. SCIs have life-changing impacts on the injured patients and their families. The rising incidence of SCIs and the corresponding treatment of patients in hospitals is followed by at least one readmission of the patient every year. This is because SCIs are not treated at once. The causes of rehospitalization include respiratory and urinary tract infections, and fractures occurring during motorcycling sports activities.
The London Spinal Cord Injury Center (LSCIC) is 1 of the 11 organizations in the UK dedicated to treating SCI patients. At the LSCIC, patients undergo a rehabilitation program that takes years and is best delivered through lifelong intervention. With rising SCI and the availability of a well-developed infrastructure in treatment centers providing rehabilitation programs, demand for spinal cord stimulation devices is surging in specialized or rehab centers, which fuels the market growth.
Future Trend
Regenerative and Innovative Implant-Based Therapeutic Approaches
The Comunidad de Madrid claims to be the first in the EU to provide treatments for SCIs using cell and regenerative therapy. Regenerative medicine includes the extraction of mesenchymal stem cells from patients suffering SCIs, treating the extracted cells in a cell production room, and then injecting the regenerated cells at the exact site of SCI or into the cerebrospinal fluid (CSF). Thus, this technique serves as a personalized treatment for SCIs.
The European Commission published a report in September 2022 revealing that scientists are developing new ways to treat SCI using graphene-based implants and virtual reality (VR) illustrations to help improve stroke recovery. They are trying these new approaches to reverse nerve damage, while some researchers are attempting to reshape the architecture of the spinal cord in situ by using engineered materials such as graphene. Graphene is used to create a 3D structure that would skillfully mimic the morphology of the spinal cord. Therefore, the use of such innovative engineered materials and implants successfully exhibits the treatment potential in patients suffering from SCIs. The regenerative and innovative implant-based therapeutic approaches are emerging as new trends in the Europe and the Middle East spinal cord stimulation devices for chronic pain market.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONEurope and Middle East Spinal Cord Stimulation Devices for Chronic Pain Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope
Product-Based Insights
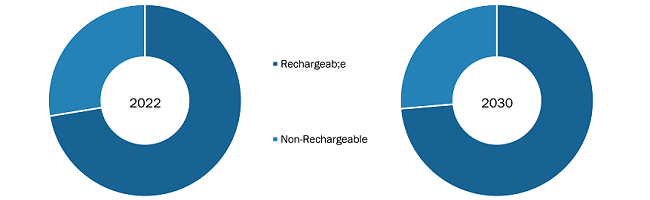
Based on product type, the spinal cord stimulation devices for chronic pain market is bifurcated into rechargeable and non-rechargeable. The rechargeable segment held the largest market share in 2022, and the same segment is expected to record a significant CAGR during 2022–2030. A spinal cord stimulator, an implantable device, sends low-magnitude electrical signals directly into the spinal cord to offer pain relief. Spinal cord stimulation is mostly employed after nonsurgical pain treatments fail to provide sufficient relief. Several top manufacturers have launched innovative rechargeable spinal cord stimulation devices in the past. In December 2022, Abbott received approval from the US Food and Drug Administration (FDA) for the world's smallest implantable and rechargeable spinal cord stimulation system indicated for chronic pain.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Application-Based Insights
Based on application, the spinal cord stimulation devices for chronic pain market is segmented as failed back syndrome, complex regional pain syndrome, degenerative disk disease, and others. The complex regional pain syndrome segment held the largest market share in 2022, and the same segment is anticipated to register the highest CAGR during 2022–2030. Complex regional pain syndrome (CRPS) involves prolonged pain and inflammation occurring due to an injury or other medical events such as surgery, trauma, stroke, and heart attack. CRPS can occur anywhere in the body, usually affecting the arm, leg, hand, or foot. It is more likely to occur in females and can occur in anyone at any age, with the inflammation approaching a peak after 40 years of age. The majority of CRPS cases are caused due to damaged or improperly functioning small peripheral C-fiber nerve fibers, which carry pain messages to the brain. CRPS often goes undiagnosed with the development of tiny clots, sometimes blocking blood flow to the nerve and injuring it.
End User-Based Insights
In terms of end user, the spinal cord stimulation devices for chronic pain market is categorized into hospitals, ambulatory surgery centers, and others. The hospital segment held a larger share of the market in 2022 and is anticipated to register a higher CAGR during 2022–2030.
Spinal cord stimulation devices are widely adopted in hospitals to treat patients suffering from SCIs. These devices are implanted via a minor surgery that is often carried out as an outpatient procedure, followed by an overnight stay of patients in the hospital. The hospital infrastructure is well-equipped to successfully use implantable devices such as spinal cord stimulators, which transmit mild electrical pulses directly into the nerve fibers of the patient after implantation, resulting in reduced chronic pain.
Spinal Cord Stimulation Devices for Chronic Pain Market, by Product Type – 2022 and 2030
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Regional Analysis
The Europe spinal cord stimulation devices for chronic pain market is segmented into Sweden, Poland, Belgium, Czech Republic, Greece, Ireland, France, the UK, Switzerland, Italy, Spain, the Netherlands, Slovakia, Germany, and the Rest of Europe. The Middle East spinal cord stimulation devices for chronic pain market is subsegmented into Saudi Arabia, Turkey, Israel, the UAE, Qatar, and Rest of the Middle East. In the EU, Germany contributes a significant share of the spinal cord stimulation devices for chronic pain market. As per the NIH, SCIs are more prevalent in Germany as per the NIH report. Therefore, an increasingly large number of SCI-associated health injuries are sent to SCI centers for rehab programs. These specialized centers are spread across the country, and they provide services such as physical, occupational, and sports therapies as well as physical medicine. Heidelberg University Hospital is an internationally recognized specialized center providing outpatient and in-patient treatments for patients suffering from SCIs. Such innovative treatments provided in specialized centers trigger the demand for spinal cord stimulation devices in Germany.
In the Middle East, Saudi Arabia accounts for a major share of the spinal cord stimulation devices for chronic pain market. According to the 2019 report from the International Journal of Medical Research & Health Sciences, Saudi Arabia has one of the world's highest rates of SCI cases. Such a high burden of SCIs is mainly due to rising road traffic accidents (RTAs). For example, 43.9% of male SCI patients in the country had a cervical injury, followed by 40.4% and 3.5% who suffered from a thoracic injury and a lumbar injury, respectively. Also, physical and psychological distress is claimed to be a prime cause of SCIs among patients in Saudi Arabia. However, early management and rehabilitation can mitigate the long-term complications of SCIs, including hospital stay duration, with prevention as the mainstay of patient care. Various rehabilitation centers have been constructed in Saudi Arabia, as a part of intrinsic modern healthcare delivery services, to overcome challenges posed by a high incidence of SCIs. There are several rehabilitation hospitals/centers in the Kingdom of Saudi Arabia (KSA), mainly in the large cities. These include the Rehabilitation Unit of Prince Sultan Military Medical City of Riyadh, Rehabilitation Unit of King Abdulaziz Medical City, National Guard (Riyadh), Rehabilitation Hospital of King Fahad Medical City (Riyadh), King Saud Medical Complex, Rehabilitation Hospital of Al-Hada Military Hospital (Taif), and Riyadh Care Hospital. With the rising SCI cases among Saudi people, progress in research is expected to result in the rollout of appropriate management programs, effective implementation of primary prevention strategies, and proper allocation of health resources for traumatic conditions.
Leading companies in the Europe and Middle East spinal cord stimulation devices for chronic pain market, which are profiled in this market study, include Boston Scientific Corp, Abbott Laboratories, Medtronic Plc, Curonix LLC, Biotronik SE & Co KG, Nevro Corp, Nalu Medical Inc, Cirtec Medical Corp, and Synapse Biomedical Inc.
- In September 2020, Boston Scientific announced the launch of the WaveWriter Alpha portfolio of spinal cord stimulator (SCS) Systems in the European market. The portfolio comprises four MRI, Bluetooth-enabled implantable pulse generators (IPGs), along with additional personalization options based on patients’ needs. These systems are available in rechargeable/non-rechargeable options which cover multiple areas of pain.
- In October 2023, Boston Scientific Corp won US FDA approval for the WaveWriter Alpha SCS systems for the treatment of painful diabetic peripheral neuropathy (DPN), a disorder that can affect the lower limbs of the body.
- In August 2022, Abbott Laboratories received approval from the US FDA for its Proclaim Plus SCS system to treat chronic pain. The device is enabled with the BurstDR stimulation technology to deliver superior pain relief. It features FlexBurst360 therapy that offers pain coverage across the trunk and limbs, alongside enabling programming that is adjustable as per the patient's evolving therapeutic needs.
Company Profiles
- Boston Scientific Corp
- Abbott Laboratories
- Medtronic Plc
- Curonix LLC
- Biotronik SE & Co KG
- Nevro Corp
- Nalu Medical Inc
- Cirtec Medical Corp
- Synapse Biomedical Inc
Europe and Middle East Spinal Cord Stimulation Devices for Chronic Pain Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 892.66 Million |
| Market Size by 2030 | US$ 1,573.67 Million |
| CAGR (2022 - 2030) | 7.3% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For