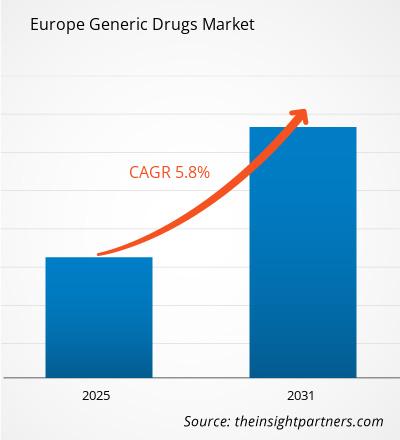
预计到2031年,欧洲仿制药市场规模将从2024年的779.5965亿美元增长至1140.3233亿美元。该市场预计在2025年至2031年期间的复合年增长率为5.8%。
执行摘要及欧洲仿制药市场分析:
得益于完善的医疗基础设施、人口老龄化以及对创新疗法的持续投入,欧洲仿制药市场有望实现强劲增长。欧洲仍然是制药和生物技术公司的聚集地,这些公司正积极研发治疗糖尿病和癌症等慢性疾病的疗法。据世界卫生组织统计,截至2024年7月,世卫组织欧洲区域约有6400万成年人和约30万儿童及青少年患有糖尿病,其中三分之一的病例未被确诊——这凸显了对价格合理的治疗方案(例如仿制药)的迫切需求。到2045年,十分之一的欧洲人可能患有糖尿病,而欧洲目前已是全球1型糖尿病负担最重的地区。癌症是另一个主要的健康问题,世卫组织报告称,2022年欧洲新增癌症病例超过447万例,癌症相关死亡病例约200万例。随着人口老龄化和慢性病发病率的上升,对经济有效的替代疗法的需求日益增长。
根据您的需求定制此报告
您可以免费获得任何报告的定制服务,包括本报告的部分内容、国家/地区层面的分析、Excel 数据包,以及面向初创企业和高校的优惠折扣。
欧洲仿制药市场:战略洞察
-
获取本报告的主要市场趋势。这份免费样品将包含数据分析,内容涵盖市场趋势、估算和预测等。
欧洲仿制药市场细分分析:
促成欧洲仿制药市场分析的关键细分市场包括分子类型、适应症、类型和分销渠道。
- 根据分子类型,欧洲仿制药市场可分为抗抑郁药、抗组胺药、镇痛药、抗生素、抗病毒药、利尿剂和其他药物。2024年,抗生素类药物占据了最大的市场份额。
- 按适应症划分,欧洲仿制药市场可分为代谢性疾病、癌症、免疫系统疾病、呼吸系统疾病、心血管疾病、神经系统疾病、罕见病和其他疾病。2024年,癌症领域占据了最大的市场份额。
- 根据类型,欧洲仿制药市场分为处方药和非处方药。2024年,处方药市场份额更大。
- 按销售渠道划分,欧洲仿制药市场可分为医院药房、零售药房和在线药房。2024年,医院药房占据了最大的市场份额。
欧洲仿制药市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2024年市场规模 | 779.5965亿美元 |
| 到2031年市场规模 | 1140.3233亿美元 |
| 复合年增长率(2025-2031年) | 5.8% |
| 史料 | 2021-2023 |
| 预测期 | 2025-2031 |
| 涵盖部分 |
按分子类型
|
| 覆盖地区和国家 |
欧洲
|
| 市场领导者和主要公司简介 |
|
欧洲仿制药市场参与者密度:了解其对商业动态的影响
受消费者偏好变化、技术进步以及消费者对产品益处认知度提高等因素推动,终端用户需求不断增长,欧洲仿制药市场正快速发展。随着需求的增长,企业不断拓展产品线、创新以满足消费者需求并把握新兴趋势,从而进一步推动市场增长。
- 获取欧洲仿制药市场主要参与者概览
欧洲仿制药市场展望
仿制药市场迎来重大机遇,这得益于医疗体系的数字化转型和公共采购平台的扩展,尤其是在发展中国家和中等收入国家。随着各国政府和保险公司日益重视以价值为导向的医疗模式并致力于控制成本,市场对高质量、低成本的品牌药替代品的需求也日益增长。这一趋势为仿制药生产商提供了融入国家医疗供应链、赢得大宗招标项目以及签订长期合同的机会。
此外,电子药房和远程医疗平台的兴起为仿制药开辟了新的零售渠道,尤其是在服务不足的偏远地区。投资数字化分销、确保符合当地监管规定并参与公私合作的企业可以利用这些趋势扩大其市场覆盖范围,并提升消费者对仿制药作为一线疗法的信任度。
欧洲仿制药市场国别分析
按国家划分,欧洲仿制药市场包括英国、德国、法国、意大利、西班牙和欧洲其他地区。2024年,欧洲其他地区占据了最大的市场份额。
挪威、丹麦、瑞典、波兰、乌克兰、罗马尼亚、比利时和捷克共和国是欧洲其他地区仿制药市场的主要国家。由于医疗保健需求不断增长、成本控制政策以及慢性病负担日益加重,该地区仿制药市场正经历稳步增长。这些国家受益于完善的医疗保健基础设施、不断增长的生物技术投资以及公共机构和私营部门之间的积极合作。在各国医疗保健预算面临日益增长的压力之际,仿制药正被视为确保药品价格合理且可持续供应的战略解决方案。荷兰和比利时等在欧盟医药生态系统中扮演核心角色的国家,正日益支持使用仿制药和生物类似药来控制治疗成本,同时保持高标准的医疗服务。癌症和肝炎等慢性病患病率的上升是推动仿制药市场需求的关键因素。根据欧盟统计局的数据,2021年欧盟多个国家超过26%的死亡病例归因于癌症——丹麦为28.2%,爱尔兰为27.7%,斯洛文尼亚为27.1%,荷兰为26.6%。这些国家也报告了大量新增癌症病例:丹麦48,840例,爱尔兰31,242例,斯洛文尼亚14,402例,荷兰132,319例。这些数据凸显了对经济有效的治疗方案的需求,仿制肿瘤药物在国家医疗体系中发挥着日益重要的作用。与此同时,当地企业正在加强低成本药物研发的创新。例如,比利时的AstriVax Therapeutics公司虽然主要专注于疫苗研发,但也展现了该地区的科研实力。尽管AstriVax等公司并不直接生产仿制药,但它们凸显了该地区强大的生物技术环境,这种环境在不久的将来也可用于仿制生物制剂和生物类似药的研发。
欧洲仿制药市场公司概况
市场上的主要参与者包括梯瓦制药工业有限公司(Teva Pharmaceutical Industries Ltd)、维亚特里斯公司(Viatris Inc)、瑞迪博士实验室有限公司(Dr. Reddy's Laboratories Ltd)、诺华公司(Novartis AG)、太阳制药工业有限公司(Sun Pharmaceutical Industries Ltd)、艾伯维公司(AbbVie Inc)、阿斯利康公司(AstraZeneca Plc)、赛诺菲公司(Sanofi SA)、阿拉宾度制药有限公司(Aurobindo Pharma Ltd)和格兰马克制药有限公司(Glenmark Pharmaceuticals Ltd)等。这些企业正采取各种战略,例如扩张、产品创新以及并购,以向消费者提供创新产品并提高市场份额。
欧洲仿制药市场研究方法:
本报告中呈现的数据收集和分析遵循以下方法:
二手研究
研究过程始于全面的二手资料研究,利用内部和外部资源收集每个市场的定性和定量数据。常用的二手资料来源包括但不限于:
- 公司网站、年度报告、财务报表、券商分析和投资者演示文稿。
- 行业贸易期刊及其他相关出版物。
- 政府文件、统计数据库和市场报告。
- 针对在该市场运营的公司的相关新闻文章、新闻稿和网络直播。
笔记:
公司概况部分包含的所有财务数据均已标准化为美元。对于以其他货币报告的公司,相关数据已使用相应年份的汇率转换为美元。
初步研究
Insight Partners每年都会对行业利益相关者和专家进行大量一手访谈,以验证其数据分析并获得宝贵见解。这些研究访谈旨在:
- 验证并完善二手研究结果。
- 提升分析团队的专业技能和市场理解能力。
- 深入了解市场规模、趋势、增长模式、竞争动态和未来前景。
初步研究通过电子邮件互动和电话访谈进行,涵盖不同地区的各个市场、类别、细分市场和子细分市场。参与者通常包括:
- 行业利益相关者:副总裁、业务拓展经理、市场情报经理和全国销售经理
- 外部专家:估值专家、研究分析师和具有行业特定专业知识的关键意见领袖
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 欧洲仿制药市场
获取免费样品 - 欧洲仿制药市场