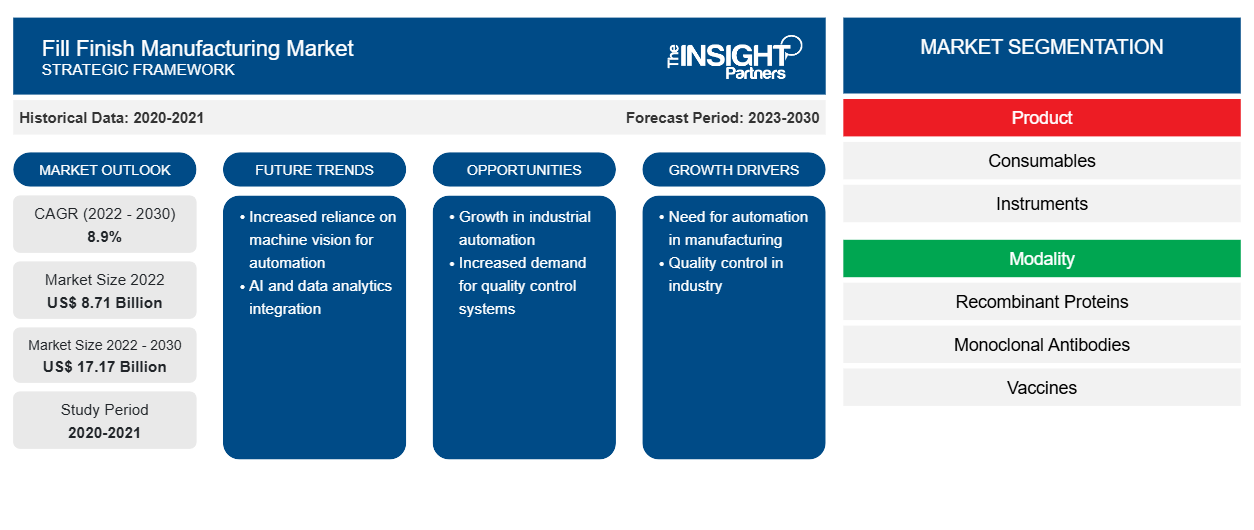
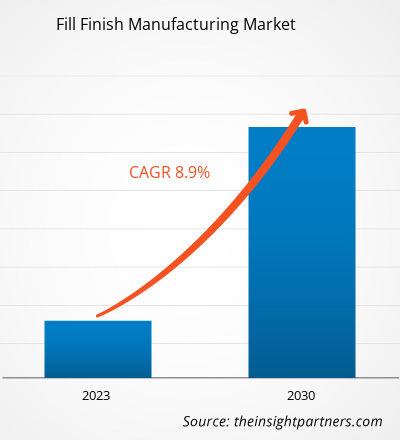
[研究报告] 填充制造市场规模预计将从 2022 年的 87.0558 亿美元增长到 2030 年的 171.6541 亿美元;预计 2022 年至 2030 年的复合年增长率为 8.9%。
市场洞察和分析师观点:
用于无菌灌装的一次性使用系统 (SUDS) 和一次性使用系统 (SUS) 为制药商节省了时间,从而使他们能够简化运营并缩短项目进度。采用 SUDS 还有助于他们确保法规遵从性,并带来环境、健康和安全 (EHS) 效益以及设施设计优势。此外,SUS 通过简化批次末期分解和净化操作来加速转换灌装操作;净化操作的简化涉及消除耗时的清洁和准备步骤。SUS 现在正用于灌装操作。批量生物技术操作已成功部署一次性系统用于缓冲液和培养基制备。他们目前正在实施新的一次性技术,例如发酵和色谱系统。技术的最新进步使 SUDS 对无菌操作越来越有吸引力。例如,一次性生产线非常适合蠕动泵可以传输的批量相对较小的肠外溶液产品(50-500L)。因此,一次性系统在无菌灌装生产中的使用增加将为灌装生产市场带来未来增长
增长动力和挑战:
生物制剂占畅销药物的绝大部分,是增长最快的制药行业领域之一。自约 30 年前推出重组蛋白疗法以来,整个生物制剂市场每年的增长率超过 12%。此外,目前正在开发 5,000 多种生物制药候选产品。尽管生物制药提供了可观的利润空间,但高昂的开发成本和复杂的生产方案是这些药物干预赞助商的主要担忧。因此,一些初创公司和制药巨头已开始将其业务运营的不同流程外包给合同服务提供商。此外,将工作外包给合同制造组织 (CMO) 和合同开发和制造组织 (CDMO) 可以减少资本投资的要求,提供更大的生产能力,缩短产品上市时间,并降低与产品商业化相关的风险。
灌装完成是生产过程的最后一步,也是药品制造最关键的阶段之一。生物制剂需要特殊的程序和设备进行灌装完成操作,以确保产品的完整性和安全性。因此,对生物制剂的需求增加导致了对灵活的无菌灌装完成技术的同等需求。制药商与合同服务提供商合作,利用他们在最新灌装完成技术方面的经验和专业知识。目前,大约有 180 家公司为各种生物制剂提供灌装完成制造服务。这些服务分布在不同地区的合同制造公司的 350 多家灌装完成工厂中。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
填充饰面制造市场:战略洞察
-
获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
此外,许多服务提供商最近收购了其他市场参与者,以增强其服务产品。例如,赛诺菲已将其生物制剂的生产外包给勃林格殷格翰。此外,艾伯维的业务已成功开发和交付药品超过 130 年;每年在公司全球网络中灌装超过 1400 万支小容量肠外注射剂 (SVP),灌装的产品分布在约 175 个国家/地区。得益于完全整合的科学和技术集团,艾伯维支持生物制剂药物 (DS) 和药物产品 (DP) 的开发,还拥有良好的审计和监管记录,并取得了临床和商业上的成功。凭借爱尔兰最先进的无菌生产线,特别是液体和冻干生物小瓶,以及与 Allergan 的 SVP 网络的整合,艾伯维进一步扩大了其能力和产能,每年超过 4000 万单位。因此,对生物制剂的需求不断增长正在推动市场增长。
制药外包的作用已从业务的一小部分转变为不可或缺的一部分。随着产品线的不断扩大、持续的价格压力和新兴市场的出现,制药行业正在发生转型。随着亚太地区服务提供商数量的不断增加,CMO 市场的竞争也愈演愈烈。美国和欧洲的小型 CMO 正在努力跟上激烈的竞争,最终,大型制药公司或其他 CMOS 正在收购它们。例如,2020 年 2 月,CMO Altasciences 收购了 Alliance Contract Pharma。此次收购为 Altascience 的产品组合增加了小分子合同制造和分析服务。尽管整合正在进行中,但 CMO 市场仍然分散,只有少数公司实现了全球覆盖和规模。
此外,由于需要大量投资才能在内部拥有这些能力,因此对抗体药物结合和高效药物制造等细分领域的 CMO 服务的需求很高。然而,许多制药公司已经开始收购这些设施,以获得相对于竞争对手的竞争优势。这导致这些服务的价格上涨,使外包成为初创公司和小型制药公司的艰难选择。
报告细分和范围:
灌装完成制造市场根据产品、方式和最终用户进行细分。根据产品,灌装完成制造市场分为消耗品和仪器。消耗品部分进一步细分为预充式注射器、玻璃瓶/塑料瓶、药筒等。根据方式,灌装完成制造市场分为重组蛋白、单克隆抗体、疫苗、细胞疗法和生物疗法、基因疗法等。就最终用户而言,灌装完成制造市场分为合同制造组织、生物制药公司和其他。根据地理位置,灌装完成制造市场细分为北美(美国、加拿大和墨西哥)、欧洲(英国、德国、法国、意大利、西班牙和欧洲其他地区)、亚太地区(中国、韩国、日本、澳大利亚、印度和亚太其他地区)、中东和非洲(阿联酋、沙特阿拉伯、南非和中东和非洲其他地区)以及南美洲和中美洲(巴西、阿根廷和南美洲和中美洲其他地区)。
节段分析:
根据产品,灌装制造市场分为消耗品和仪器。消耗品部分进一步分为预充式注射器、玻璃瓶/塑料瓶、药筒和其他。消耗品部分分为药筒、药瓶、预充式注射器和其他消耗品,如安瓿瓶、瓶子、袋子和一次性系统。消耗品部分在灌装制造市场中占有相当大的市场份额;由于与仪器相比更换率高,预计在预测期内将经历类似的增长趋势。此外,消耗品的保质期较短,通常需要大量使用。预充式注射器的采用率不断提高、药瓶在冻干中的应用多种多样以及灌装外包的增加是推动消耗品部分发展的关键因素。
根据方式,灌装完成制造市场分为重组蛋白、单克隆抗体、疫苗、细胞疗法和生物疗法、基因疗法等。疫苗灌装完成制造是生产过程中的关键阶段。它涉及用准备好的疫苗填充小瓶并确保精确的数量。此步骤需要严格的质量控制以保持有效性和安全性。装满后,小瓶要密封和包装。这一细致的过程在向全球人口提供疫苗、通过满足质量标准和监管要求来保障公众健康方面发挥着关键作用。此外,与疫苗灌装完成制造相关的关键发展不断增加,正在推动市场增长。例如,2023 年 5 月,安大略省领先的无菌注射剂合同开发和制造组织 (CDMO) Novocol Pharma 和率先开发信使 RNA (mRNA) 疗法和疫苗的生物技术公司 Moderna, Inc. 宣布了一项长期协议,对预计将在加拿大生产的 mRNA 呼吸道疫苗进行无菌灌装完成、贴标和包装。生产周期的最后阶段称为灌装-完成无菌制造,包括将疫苗药物产品放入小瓶并准备使用。此外,与 Novocol Pharma 的合作将增加 Moderna 位于拉瓦尔的 mRNA 工厂生产的疫苗的灌装能力。根据规划和监管许可,该工厂预计将于 2024 年底投入运营
根据最终用户,灌装完成制造市场分为合同制造组织、生物制药公司和其他。2022 年,合同制造组织部分占据了最大的市场份额,预计在预测期内将实现最高的复合年增长率。合同制造组织 (CMO) 通过最大限度地减少设施投资要求和药物开发成本,从而改善净现金流,使制药公司受益。外包是一种更便宜的方法,可以提高制造流程的效率。此外,它还允许制药和生物技术公司将资源重新分配到其他必要的领域。过去几年,CMO 一直被认为是一个小众行业,它为制药公司提供了额外的制造能力或特定服务。药品制造失败的不断增加导致 CMO 数量增加。制药公司传统上拥有专门用于开发创新药物的制造设施。然而,为了降低产能过剩的风险,对制造外包的需求一直在不断上升。
许多制药公司已开始重新关注其核心能力,例如研发,从而导致内部制造能力的剥离,导致制造越来越依赖 CMO。此外,CMO 形式的额外产能减轻了供应短缺的风险。他们还为制药公司提供其他场地,以实施多站点供应策略并保留备用产能。例如,2020 年,三星生物制剂和 GI Innovation 签署了免疫化疗合同。根据该协议,三星生物制剂为 GI Innovation 提供从 f 细胞系开发到 I 期药物物质生产的服务。
区域分析:
根据地理位置,灌装制造市场细分为北美、欧洲、亚太地区、中东和非洲以及南美和中美。北美是灌装制造市场增长的最大贡献者。加拿大是生物制药行业发展最快的国家之一。多家国际公司正在投资加拿大生物制药行业。在加拿大,生物制药公司的数量正在大幅增加。例如,不列颠哥伦比亚省温哥华拥有大多数生物制药公司,每年创造 9030 万美元(1.2 亿加元)的收入。全国约有 35-40 家国内生物制药公司。加拿大的一些生物制药公司包括 Inex Pharmaceuticals、Quest Vitamins、StressGen Biotechnologies、Stanley Pharmaceuticals、QLT Phototherapeutics 和 Stemcell Technologies。各种公共和私人研究机构包括分子医学和治疗学中心、不列颠哥伦比亚大学生物技术实验室、不列颠哥伦比亚癌症研究中心、加拿大艾滋病毒试验网络和疫苗评估中心。因此,该国生物制药行业的发展将在预测期内促进市场增长。
预计亚太地区将成为未来几年增长最快的市场。在亚太地区,中国是最大的灌装制造市场。市场的增长主要归因于中国灌装制造工艺技术的不断进步、市场参与者的不断发展、生物制药行业的扩张以及慢性病的日益流行。中国有 500 多家生物制品/生物制药公司。大多数参与研发的公司都是由海归或西方/合资公司创立的。尽管估计数字相差很大,但分析师认为,中国政府每年通过资助计划在生物技术研发上投入超过 6 亿美元。中国国家和地方政府还投资于投资 IT 企业的准风险投资公司。市场参与者正在通过有机和无机增长战略扩大业务。例如,药明生物于 2022 年 6 月在中国无锡开设药品工厂,将预灌封注射器 (PFS) 的产能提高到每年 1700 万支。药明生物是一家合同开发制造组织 (CDMO),其运营的最新 DP 工厂名为 DP5,拥有一条先进的隔离器灌装线,可提供可靠、连续的灌装服务。据该公司称,这为 PFS 提供了多种容量输送选项,包括 1.25 mL、3 mL、1 mL 和 1 mL。
填充饰面制造市场区域洞察
Insight Partners 的分析师已详细解释了预测期内影响填充饰面制造市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的填充饰面制造市场细分和地理位置。
- 获取填充制造市场的区域特定数据
填充制品制造市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2022 年市场规模 | 87.1亿美元 |
| 2030 年市场规模 | 171.7亿美元 |
| 全球复合年增长率(2022 - 2030 年) | 8.9% |
| 史料 | 2020-2021 |
| 预测期 | 2023-2030 |
| 涵盖的领域 |
按产品
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
填充饰面制造市场参与者密度:了解其对业务动态的影响
填充饰面制造市场正在快速增长,这得益于最终用户需求的不断增长,这些需求源于消费者偏好的不断变化、技术进步以及对产品优势的认识不断提高等因素。随着需求的增加,企业正在扩大其产品范围,进行创新以满足消费者的需求,并利用新兴趋势,从而进一步推动市场增长。
市场参与者密度是指在特定市场或行业内运营的企业或公司的分布情况。它表明在给定市场空间中,相对于其规模或总市场价值,有多少竞争对手(市场参与者)存在。
在填充制造市场运营的主要公司有:
- IMA 工业自动化机械有限公司
- 尼普洛医疗欧洲有限公司
- 达拉工业机械公司
- 格罗宁根有限公司
- 新加坡元南非
免责声明:上面列出的公司没有按照任何特定顺序排列。
- 获取填充饰面制造市场顶级关键参与者概览
竞争格局和重点公司:
IMA Industria Macchine Automatiche SpA、Nipro Medical Europe NV、Maquinaria Industrial Dara SL、Groninger and Co GmbH、SGD SA、Optima Packaging Group Gmbh、NNE AS、Stevanato Group SpA、Syntegon Technology GmbH、West Pharmaceutical Services Inc、Gerresheimer AG、Schott AG 和 Becton Dickinson and Co 是灌装制造市场中的几家知名企业。这些公司专注于扩大服务范围,以满足全球日益增长的消费者需求。它们的全球业务使它们能够服务于大量客户,从而扩大其市场份额。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 填充饰面制造市场
获取免费样品 - 填充饰面制造市场