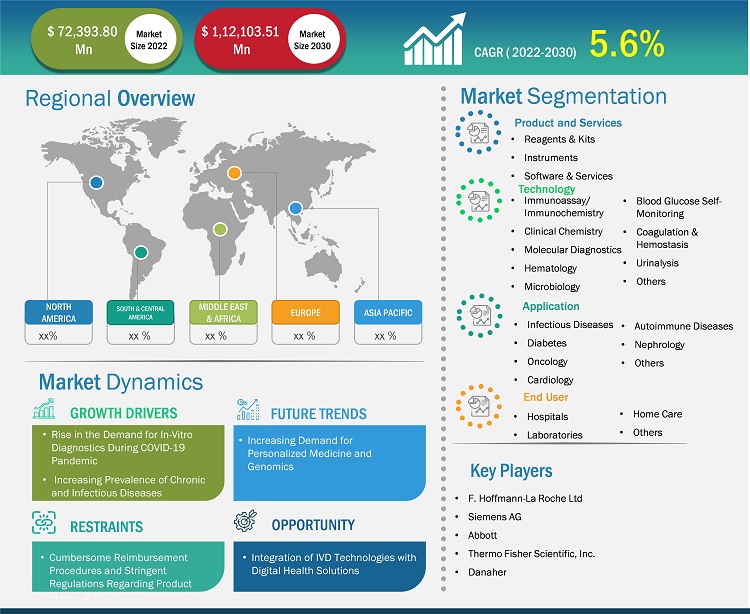
[Research Report] The in-vitro diagnostic market size was valued at US$ 72,393.80 million in 2022 and is expected to reach US$ 1,12,103.51 million by 2030; the market is estimated to register a CAGR of 5.6% from 2022 to 2030
Market Insights and Analyst View:
In-vitro diagnostics includes laboratory testing and medical devices used to analyze biological samples, such as urine, blood, or tissues, obtained from a human body. These tests play a vital role in monitoring and diagnosing various medical conditions. The increasing prevalence of chronic diseases, technological advancements, and the growing geriatric population are the noteworthy factors driving the in-vitro diagnostics market growth. Advancements in molecular diagnostics empower efforts made by research groups and healthcare workforces across the world to improve personalized treatment plans based on the genetic makeup of individuals. Growth opportunities in developing regions due to progressing infrastructure and health-related awareness can also be associated with the projected growth of the in-vitro diagnostics market during 2022–2030.
Growth Drivers:
According to CDC data, coronary artery disease (CAD) is the most common cause of adult mortality. Approximately 375,476 people died due to CAD in 2021. Estimates published in the 2022 National Diabetes Statistics Report by the World Health Organization state that ~422 million people worldwide have diabetes, the majority of which are living in low- and middle-income countries. Moreover, 1.5 million deaths are directly related to diabetes each year. Overweight, genetic conditions, aging, sedentary lifestyle, etc., are boosting the prevalence of diabetes. According to estimations published in a Cancer Research UK report, ~18.1 million new cancer cases were reported worldwide in 2020, and the number is expected to increase to 28 million by 2040. Hence, the rising prevalence of infectious and chronic diseases such as cardiovascular diseases, cancer, diabetes, and respiratory conditions drives the growth of the in-vitro diagnostics market.
IVD is used in clinical, laboratory, and outpatient settings with the aim specifically to help in the detection of diseases and, consequently, aid in the selection of appropriate treatment protocols. The integration of IVD technologies with digital health solutions is gaining traction globally. Data analytics, artificial Intelligence, and remote monitoring enhance the value of diagnostic tests, leading to better patient management and outcomes. IVD technologies integrated with digital health solutions can be incorporated into clinical decision support systems. As recognized by the WHO, digital health solutions could help detect diseases. Artificial intelligence health bots and similar other emerging solutions may present opportunities for patient care and address challenges such as high cost and time requirements. In diagnostics based on genomic testing, deep learning can identify cancer cells, determine their type, and predict what mutations may occur in a tumor from images of a specific sample. Artificial intelligence and machine learning (AI/ML) in in-vitro diagnostics are revolutionizing medical device development. These modern diagnostic systems facilitate diagnosis based on digital image analysis, thereby improving healthcare decision-making. Smart diagnostics are extremely scalable IVD solutions that use artificial intelligence to perform better than lab-based diagnostics at a fraction of the price. Additionally, this type of diagnostics can derive emergent features through unique chemical and biological signature detection and analysis. Thus, the integration of IVD with digital health technologies is likely to offer lucrative opportunities to the in-vitro diagnostics market in the coming years.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
In-Vitro Diagnostic Market: Strategic Insights
Market Size Value in US$ 72,393.80 million in 2022 Market Size Value by US$ 1,12,103.51 million by 2030 Growth rate CAGR of 5.6% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
In-Vitro Diagnostic Market: Strategic Insights

| Market Size Value in | US$ 72,393.80 million in 2022 |
| Market Size Value by | US$ 1,12,103.51 million by 2030 |
| Growth rate | CAGR of 5.6% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Report Segmentation and Scope:
The global in-vitro diagnostics market is segmented into product & service, technology, and application. Based on product & service, the market is categorized into reagents & kits, instruments, and software & services. In terms of technology, the in-vitro diagnostics market is segmented into immunoassay/immunochemistry, clinical chemistry, molecular diagnostics, microbiology, blood glucose self-monitoring, coagulation and hemostasis, hematology, urinalysis, and others. The in-vitro diagnostics market, by application, is fragmented into infectious diseases, diabetes, oncology, cardiology, autoimmune diseases, nephrology, and others. The in-vitro diagnostic market, by end user, is segmented into hospitals, laboratories, home care, and others. The in-vitro diagnostics market, based on geography, is segmented into North America (the US, Canada, and Mexico), Europe (Germany, France, Italy, the UK, Spain, and Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and Rest of Asia Pacific), the Middle East & Africa (South Africa, Saudi Arabia, the UAE, and Rest of the Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America).
Segmental Analysis:
Based on products & services, the in-vitro diagnostic market is segmented into reagents & kits, instruments, and software & services. In 2022, the reagents & kits segment held the largest share of the market due to the popularity of self-test kits and POC devices. The projected growth of the market for reagents and kits is attributed to the tremendous popularity of self-test kits and POC devices, and a rise in availability and adoption automated instruments that simplify jobs and provide accurate results. The increasing cases of viral and fungal infections with inadequate hygienic conditions promote reagent use. Reagents and kits, in addition to other consumables, are frequently used in research processes. In-vitro diagnostics tests are performed on urine, blood, stool, and tissue samples to diagnose various conditions, from mild infections to life-threatening diseases such as cancer. Abbott, F. Hoffmann-La Roche Ltd, and Bio-Rad Laboratories, Inc. are among the key companies offering kits and reagents. Governments implemented mass screening programs during the COVID-19 outbreak, which propelled the in-vitro diagnostics market growth. In 2020, Abbott Laboratories ramped up its production of COVID-19 test kits, including a new tool that could enable mass COVID-19 screening.
The in-vitro diagnostic market is segmented based on application into infectious disease, diabetes, oncology, cardiology, autoimmune disease, nephrology, and others. In 2022, the infectious disease segment held the largest share of the market. The same segment is expected to register the highest CAGR in the market during 2022-2030.
Based on technology, the in-vitro diagnostics market is segmented into immunoassay/immunochemistry, clinical chemistry, molecular diagnostics, microbiology, blood glucose self-monitoring, coagulation and hemostasis, hematology, urinalysis, and others. In 2022, the immunoassay/ immunochemistry segment held the largest share of the market. However, the molecular diagnostics segment is expected to register the fastest CAGR during 2022–2030 owing to the launch of novel products and continuous technological evolution. Moreover, COVID-19 has positively impacted immunoassay and molecular diagnostics.
The In-vitro diagnostics market, based on end user, is segmented into hospitals, laboratories, home care, and others. In 2022, the hospitals segment held the largest market share. Additionally, the continued expansion of healthcare infrastructure would lead to improvements in current hospital facilities, which is likely to trigger the demand for IVD tests performed in these facilities. The growing number of hospital admissions, coupled with the spur in the prevalence of chronic diseases, is expected to fuel the growth of the in-vitro diagnostics market for the hospital segment during 2022–2030. Developing countries are witnessing a huge demand for advanced hospital settings to cope with the increasing patient pool and rising public health concerns. Thus, a growing number of hospitals are anticipated to boost the adoption of in-vitro diagnostics due to its superior benefits. Furthermore, benefits provided by hospitals, such as proper patient-centric care and availability of reimbursement facilities fuel the market growth of this segment.
Regional Analysis:
Based on geography, the in-vitro diagnostic market is divided into five key regions: North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. The market in North America has been analyzed with a prime focus on three major countries—the US, Canada, and Mexico. The US held the largest share of the North American in-vitro diagnostics market in 2022. It is estimated to hold the largest share in the in-vitro diagnostic market in North America during the forecast period. The market growth in this country is ascribed to the increasing prevalence of chronic and infectious diseases, focus on efficient disease diagnosis, and a higher need for advanced healthcare systems. Chronic diseases such as cancer and cardiovascular disease are the major causes of disabilities and death in the US. As per the National Center for Chronic Disease Prevention and Health Promotion, 6 in 10 people in the country have at least 1 chronic disease. In 2021, ~18.2 million adults aged 20 and above had coronary artery disease (CAD) in the US, as per the report by the Centers for Disease Control and Prevention (CDC). CAD is the leading cause of death among people in the country.
The American Hospital Association also estimates ~133 million people have at least 1 chronic disease, and the number is expected to reach 170 million by 2030. The high incidence of chronic diseases results in a huge demand for diagnostic procedures, which, in turn, drives the in-vitro diagnostics market in the US. Growing emphasis on preventive care and enhanced access to healthcare facilities would further boost the market growth in the coming years.
In-Vitro Diagnostic Market Opportunity:
IVD is used in clinical, laboratory, and outpatient settings with the aim specifically to help detect diseases and, consequently, aid in selecting appropriate treatment protocols. The integration of IVD technologies with digital health solutions is gaining traction globally. Data analytics, artificial intelligence, and remote monitoring enhance the value of diagnostic tests, leading to better patient management and outcomes. IVD technologies integrated with digital health solutions can be incorporated into clinical decision support systems. As recognized by the WHO, digital health solutions could help detect diseases. Artificial intelligence health bots and similar emerging solutions may present opportunities for patient care and address challenges such as high cost and time requirements. In diagnostics based on genomic testing, deep learning can identify cancer cells, determine their type, and predict what mutations may occur in a tumor from images of a specific sample. Machine learning and artificial intelligence in in-vitro diagnostics are revolutionizing medical device development. These modern diagnostic systems facilitate diagnosis based on digital image analysis, improving healthcare decision-making. Smart diagnostics are extremely scalable IVD solutions that use artificial intelligence to perform better than lab-based diagnostics at a fraction of the price. Additionally, this type of diagnostics can derive emergent features through unique chemical and biological signature detection and analysis. Thus, integrating IVD with digital health technologies is likely to offer lucrative opportunities to the in-vitro diagnostics market in the coming years.
In-Vitro Diagnostics Market Report Scope
Competitive Landscape and Key Companies:
A few of the prominent in-vitro diagnostic manufacturers operating in the global in-vitro diagnostic market are Abbott Laboratories, F. Hoffmann-La Roche Ltd, Danaher Corp, Siemens AG, Sysmex Corp, Thermo Fisher Scientific Inc, Becton Dickinson and Co, bioMerieux SA, Bio-Rad Laboratories Inc, Qiagen NV. These companies focus on new technologies, advancements in existing products, and geographic expansions to meet the increasing consumer demand worldwide and growing their product range in specialty portfolios. For instance, in October 2020, Abbott signed a non-exclusive royalty-bearing license agreement with Quanterix Corporation. Under the agreement, Abbott has gained access to Quanterix’s portfolio of bead-based technology patents for in vitro diagnostic (IVD) applications. In addition, Quanterix received an initial license fee, regulatory and launch milestones, milestone fees subject to achievements associated with Abbott’s future developments, and royalties on the sale of licensed products.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product & Service, Technology, Application, End User, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
In vitro diagnostics (IVD) are tests done on samples such as blood or tissues that have been taken from the human body. In-vitro diagnostics are specialized to detect diseases or other conditions that can be used to monitor a person's overall health to help cure, treat, or prevent diseases. Additionally, in-vitro diagnostics may also be utilized for precision medicine to identify patients who are likely to benefit from specific treatments or therapies. Therefore, these in-vitro diagnostics can include next-generation sequencing tests that scan a person's DNA to detect genomic variations.
The CAGR value of the in-vitro diagnostics market during the forecasted period of 2022-2030 is 5.6%.
Key factors that are driving the growth of this market are increasing prevalence of chronic diseases, technological advancements, and the growing geriatric population are expected to boost the market growth for the in-vitro diagnostics over the years.
The reagent & kits segment held the largest share of the market in the global in-vitro diagnostics market and held the largest market share of 79.00% in 2022.
Abbott and F.Hoffman-La-Roche are the top two companies that hold huge market shares in the in-vitro diagnostics market.
The infectious diseases segment dominated the global in-vitro diagnostics market and held the largest market share of 39.8% in 2022.
Global in-vitro diagnostics market is segmented by region into North America, Europe, Asia Pacific, the Middle East & Africa and South & Central America. Europe held the largest market share of the in-vitro diagnostics market in 2022. With several European market players focusing on research and development activities in the field of imaging technology, the regional market for in-vitro diagnostics market is likely to propel in Europe region during the forecast period.
The in-vitro diagnostics market majorly consists of the players such Abbott Laboratories, F. Hoffmann-La Roche Ltd, Danaher Corp, Siemens AG, Sysmex Corp, Thermo Fisher Scientific Inc, Becton Dickinson and Co, bioMerieux SA, Bio-Rad Laboratories Inc, and Qiagen NV.
1. Introduction
1.1 The Insight Partners Research Report Guidance
1.2 Market Segmentation
2. Executive Summary
2.1 Key Insights
2.2 In-Vitro Diagnostics Market, by Geography (US$ Million)
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. In-Vitro Diagnostics Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 PEST Analysis
5. In-Vitro Diagnostics Market - Key Industry Dynamics
5.1 Key Market Drivers
5.1.1 Increasing Prevalence of Chronic and Infectious Diseases
5.1.2 Surge in Demand for In-Vitro Diagnostics During COVID-19 Pandemic
5.2 Market Restraint
5.2.1 Insufficiency of Regulatory Frameworks and Reimbursement Policies
5.3 Market Opportunities
5.3.1 Integration of IVD with Digital Health Technologies
5.4 Future Trends
5.4.1 Increasing Demand for Personalized Medicine and Genomics
6. In-Vitro Diagnostics Market - Global Market Analysis
6.1 In-Vitro Diagnostics Market Revenue (US$ Mn), 2022 – 2030
7. Global In-Vitro Diagnostics Market – Revenue and Forecast to 2030 – by Product and Services
7.1 Overview
7.2 In-Vitro Diagnostics Market Revenue Share, by Product and Services 2022 & 2030 (%)
7.3 Reagents and Kits
7.3.1 Overview
7.3.2 Reagents and Kits: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
7.4 Software and Services
7.4.1 Overview
7.4.2 Software and Services: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
7.5 Instruments
7.5.1 Overview
7.5.2 Instruments: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8. Global In-Vitro Diagnostics Market – Revenue and Forecast to 2030 – by Technology
8.1 Overview
8.2 In-Vitro Diagnostics Market Revenue Share, by Technology 2022 & 2030 (%)
8.3 Immunoassay/ Immunochemistry
8.3.1 Overview
8.3.2 Immunoassay/ Immunochemistry: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.4 Clinical Chemistry
8.4.1 Overview
8.4.2 Clinical Chemistry: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.5 Molecular Diagnostics
8.5.1 Overview
8.5.2 Molecular Diagnostics: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.6 Microbiology
8.6.1 Overview
8.6.2 Microbiology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.7 Blood Glucose Self-Monitoring
8.7.1 Overview
8.7.2 Blood Glucose Self-Monitoring: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.8 Coagulation and Hemostasis
8.8.1 Overview
8.8.2 Coagulation and Hemostasis: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.9 Hematology
8.9.1 Overview
8.9.2 Hematology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.10 Urinalysis
8.10.1 Overview
8.10.2 Urinalysis: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
8.11 Others
8.11.1 Overview
8.11.2 Others: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9. Global In-Vitro Diagnostics Market – Revenue and Forecast to 2030 – by Application
9.1 Overview
9.2 In-Vitro Diagnostics Market Revenue Share, by Application 2022 & 2030 (%)
9.3 Infectious Disease
9.3.1 Overview
9.3.2 Infectious Diseases: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9.4 Diabetes
9.4.1 Overview
9.4.2 Diabetes: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9.5 Oncology
9.5.1 Overview
9.5.2 Oncology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9.6 Cardiology
9.6.1 Overview
9.6.2 Cardiology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9.7 Autoimmune Diseases
9.7.1 Overview
9.7.2 Autoimmune Diseases: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9.8 Nephrology
9.8.1 Overview
9.8.2 Nephrology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
9.9 Others
9.9.1 Overview
9.9.2 Others: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
10. Global In-Vitro Diagnostics Market – Revenue and Forecast to 2030 – by End User
10.1 Overview
10.2 In-Vitro Diagnostics Market Revenue Share, by End User 2022 & 2030 (%)
10.3 Hospitals
10.3.1 Overview
10.3.2 Hospitals: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
10.4 Laboratories
10.4.1 Overview
10.4.2 Laboratories: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
10.5 Homecare
10.5.1 Overview
10.5.2 Homecare: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
10.6 Others
10.6.1 Overview
10.6.2 Others: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11. In-Vitro Diagnostics Market – Revenue and Forecast to 2030 – Geographic Analysis
11.1 North America: In-Vitro Diagnostics Market
11.1.1 Overview
11.1.2 North America: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.3 North America: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.1.4 North America: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.1.5 North America: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.1.6 North America: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.1.7 North America: In-Vitro Diagnostics Market, by Country
11.1.7.1 US: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.7.1.1 Overview
11.1.7.1.2 US: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.7.1.3 US: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.1.7.1.4 US: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.1.7.1.5 US: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.1.7.1.6 US: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.1.7.2 Canada: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.7.2.1 Overview
11.1.7.2.2 Canada: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.7.2.3 Canada: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.1.7.2.4 Canada: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.1.7.2.5 Canada: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.1.7.2.6 Canada: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.1.7.3 Mexico: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.7.3.1 Overview
11.1.7.3.2 Mexico: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.1.7.3.3 Mexico: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.1.7.3.4 Mexico: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.1.7.3.5 Mexico: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.1.7.3.6 Mexico: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2 Europe: In-Vitro Diagnostics Market
11.2.1 Overview
11.2.2 Europe: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.3 Europe: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.4 Europe: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.5 Europe: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.6 Europe: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2.7 Europe: In-Vitro Diagnostics Market, by Country
11.2.7.1 Germany: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.1.1 Overview
11.2.7.1.2 Germany: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.1.3 Germany: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.7.1.4 Germany: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.7.1.5 Germany: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.7.1.6 Germany: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2.7.2 France: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.2.1 Overview
11.2.7.2.2 France: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.2.3 France: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.7.2.4 France: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.7.2.5 France: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.7.2.6 France: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2.7.3 UK: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.3.1 Overview
11.2.7.3.2 UK: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.3.3 UK: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.7.3.4 UK: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.7.3.5 UK: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.7.3.6 UK: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2.7.4 Italy: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.4.1 Overview
11.2.7.4.2 Italy: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.4.3 Italy: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.7.4.4 Italy: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.7.4.5 Italy: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.7.4.6 Italy: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2.7.5 Spain: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.5.1 Overview
11.2.7.5.2 Spain: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.5.3 Spain: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.7.5.4 Spain: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.7.5.5 Spain: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.7.5.6 Spain: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.2.7.6 Rest of Europe: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.6.1 Overview
11.2.7.6.2 Rest of Europe: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.6.3 Rest of Europe: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.2.7.6.4 Rest of Europe: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.2.7.6.5 Rest of Europe: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.2.7.6.6 Rest of Europe: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3 Asia Pacific: In-Vitro Diagnostics Market
11.3.1 Overview
11.3.2 Asia Pacific: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.3 Asia Pacific: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.4 Asia Pacific: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.5 Asia Pacific: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.6 Asia Pacific: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3.7 Asia Pacific: In-Vitro Diagnostics Market, by Country
11.3.7.1 China: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.1.1 Overview
11.3.7.1.2 China: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.1.3 China: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.7.1.4 China: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.7.1.5 China: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.7.1.6 China: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3.7.2 Japan: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.2.1 Overview
11.3.7.2.2 Japan: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.2.3 Japan: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.7.2.4 Japan: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.7.2.5 Japan: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.7.2.6 Japan: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3.7.3 India: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.3.1 Overview
11.3.7.3.2 India: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.3.3 India: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.7.3.4 India: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.7.3.5 India: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.7.3.6 India: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3.7.4 Australia: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.4.1 Overview
11.3.7.4.2 Australia: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.4.3 Australia: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.7.4.4 Australia: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.7.4.5 Australia: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.7.4.6 Australia: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3.7.5 South Korea: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.5.1 Overview
11.3.7.5.2 South Korea: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.5.3 South Korea: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.7.5.4 South Korea: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.7.5.5 South Korea: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.7.5.6 South Korea: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.3.7.6 Rest of Asia Pacific: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.6.1 Overview
11.3.7.6.2 Rest of Asia Pacific: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.3.7.6.3 Rest of Asia Pacific: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.3.7.6.4 Rest of Asia Pacific: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.3.7.6.5 Rest of Asia Pacific: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.3.7.6.6 Rest of Asia Pacific: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.4 Middle East & Africa: In-Vitro Diagnostics Market
11.4.1 Overview
11.4.2 Middle East & Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.3 Middle East & Africa: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.4.4 Middle East & Africa: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.4.5 Middle East & Africa: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.4.6 Middle East & Africa: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.4.7 Middle East & Africa: In-Vitro Diagnostics Market, by Country
11.4.7.1 South Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.1.1 Overview
11.4.7.1.2 South Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.1.3 South Africa: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.4.7.1.4 South Africa: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.4.7.1.5 South Africa: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.4.7.1.6 South Africa: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.4.7.2 Saudi Arabia: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.2.1 Overview
11.4.7.2.2 Saudi Arabia: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.2.3 Saudi Arabia: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.4.7.2.4 Saudi Arabia: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.4.7.2.5 Saudi Arabia: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.4.7.2.6 Saudi Arabia: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.4.7.3 UAE: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.3.1 Overview
11.4.7.3.2 UAE: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.3.3 UAE: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.4.7.3.4 UAE: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.4.7.3.5 UAE: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.4.7.3.6 UAE: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.4.7.4 Rest of Middle East & Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.4.1 Overview
11.4.7.4.2 Rest of Middle East & Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.4.7.4.3 Rest of Middle East & Africa: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.4.7.4.4 Rest of Middle East & Africa: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.4.7.4.5 Rest of Middle East & Africa: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.4.7.4.6 Rest of Middle East & Africa: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.5 South & Central America: In-Vitro Diagnostics Market
11.5.1 Overview
11.5.2 South & Central America: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.3 South & Central America: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.5.4 South & Central America: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.5.5 South & Central America: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.5.6 South & Central America: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.5.7 South & Central America: In-Vitro Diagnostics Market, by Country
11.5.7.1 Brazil: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.7.1.1 Overview
11.5.7.1.2 Brazil: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.7.1.3 Brazil: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.5.7.1.4 Brazil: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.5.7.1.5 Brazil: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.5.7.1.6 Brazil: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.5.7.2 Argentina: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.7.2.1 Overview
11.5.7.2.2 Argentina: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.7.2.3 Argentina: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.5.7.2.4 Argentina: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.5.7.2.5 Argentina: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.5.7.2.6 Argentina: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
11.5.7.3 Rest of South & Central America: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.7.3.1 Overview
11.5.7.3.2 Rest of South & Central America: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
11.5.7.3.3 Rest of South & Central America: In-Vitro Diagnostics Market, by Product and Services, 2020–2030 (US$ Million)
11.5.7.3.4 Rest of South & Central America: In-Vitro Diagnostics Market, by Technology, 2020–2030 (US$ Million)
11.5.7.3.5 Rest of South & Central America: In-Vitro Diagnostics Market, by Application, 2020–2030 (US$ Million)
11.5.7.3.6 Rest of South & Central America: In-Vitro Diagnostics Market, by End User, 2020–2030 (US$ Million)
12. Industry Landscape
12.1 Overview
12.2 Growth Strategies in North America In-Vitro Diagnostics Market
12.3 Organic Developments
12.3.1 Overview
12.4 Inorganic Developments
12.4.1 Overview
13. Company Profiles
13.1 Abbott Laboratories
13.1.1 Key Facts
13.1.2 Business Description
13.1.3 Products and Services
13.1.4 Financial Overview
13.1.5 SWOT Analysis
13.1.6 Key Developments
13.2 F. Hoffmann-La Roche Ltd
13.2.1 Key Facts
13.2.2 Business Description
13.2.3 Products and Services
13.2.4 Financial Overview
13.2.5 SWOT Analysis
13.2.6 Key Developments
13.3 Danaher Corp
13.3.1 Key Facts
13.3.2 Business Description
13.3.3 Products and Services
13.3.4 Financial Overview
13.3.5 SWOT Analysis
13.3.6 Key Developments
13.4 Siemens AG
13.4.1 Key Facts
13.4.2 Business Description
13.4.3 Products and Services
13.4.4 Financial Overview
13.4.5 SWOT Analysis
13.4.6 Key Developments
13.5 Sysmex Corp
13.5.1 Key Facts
13.5.2 Business Description
13.5.3 Products and Services
13.5.4 Financial Overview
13.5.5 SWOT Analysis
13.5.6 Key Developments
13.6 Thermo Fisher Scientific Inc
13.6.1 Key Facts
13.6.2 Business Description
13.6.3 Products and Services
13.6.4 Financial Overview
13.6.5 SWOT Analysis
13.6.6 Key Developments
13.7 Becton Dickinson and Co
13.7.1 Key Facts
13.7.2 Business Description
13.7.3 Products and Services
13.7.4 Financial Overview
13.7.5 SWOT Analysis
13.7.6 Key Developments
13.8 bioMerieux SA
13.8.1 Key Facts
13.8.2 Business Description
13.8.3 Products and Services
13.8.4 Financial Overview
13.8.5 SWOT Analysis
13.8.6 Key Developments
13.9 Bio-Rad Laboratories Inc
13.9.1 Key Facts
13.9.2 Business Description
13.9.3 Products and Services
13.9.4 Financial Overview
13.9.5 SWOT Analysis
13.9.6 Key Developments
13.10 Qiagen NV
13.10.1 Key Facts
13.10.2 Business Description
13.10.3 Products and Services
13.10.4 Financial Overview
13.10.5 SWOT Analysis
13.10.6 Key Developments
14. Appendix
14.1 About Us
14.2 Glossary of Terms
List of Tables
Table 1. In-Vitro Diagnostics Market Segmentation
Table 2. North America In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 3. North America In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 4. North America In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 5. North America In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 6. US In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 7. US In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 8. US In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 9. US In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 10. Canada In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 11. Canada In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 12. Canada In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 13. Canada In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 14. Mexico In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 15. Mexico In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 16. Mexico In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 17. Mexico In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 18. Europe In-Vitro Diagnostics Market, For Reagents and Kits by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 19. Europe In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 20. Europe In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 21. Europe In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 22. Germany In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 23. Germany In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 24. Germany In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 25. Germany In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 26. France In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 27. France In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 28. France In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 29. France In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 30. UK In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 31. UK In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 32. UK In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 33. UK In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 34. Italy In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 35. Italy In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 36. Italy In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 37. Italy In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 38. Spain In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 39. Spain In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 40. Spain In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 41. Spain In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 42. Rest of Europe In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 43. Rest of Europe In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 44. Rest of Europe In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 45. Rest of Europe In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 46. Asia Pacific In-Vitro Diagnostics Market, For Reagents and Kits by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 47. Asia Pacific In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 48. Asia Pacific In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 49. Asia Pacific In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 50. Death Rate Of Cancer in 2020 Globally
Table 51. China In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 52. China In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 53. China In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 54. China In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 55. Japan In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 56. Japan In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 57. Japan In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 58. Japan In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 59. India In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 60. India In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 61. India In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 62. India In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 63. Australia In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 64. Australia In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 65. Australia In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 66. Australia In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 67. South Korea In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 68. South Korea In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 69. South Korea In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 70. South Korea In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 71. Rest of Asia Pacific In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 72. Rest of Asia Pacific In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 73. Rest of Asia Pacific In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 74. Rest of Asia Pacific In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 75. Middle East & Africa In-Vitro Diagnostics Market, For Reagents and Kits by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 76. Middle East & Africa In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 77. Middle East & Africa In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 78. Middle East & Africa In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 79. South Africa In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 80. South Africa In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 81. South Africa In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 82. South Africa In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 83. Saudi Arabia In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 84. Saudi Arabia In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 85. Saudi Arabia In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 86. Saudi Arabia In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 87. UAE In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 88. UAE In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 89. UAE In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 90. UAE In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 91. Rest of Middle East & Africa In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 92. Rest of Middle East & Africa In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 93. Rest of Middle East & Africa In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 94. Rest of Middle East & Africa In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 95. South & Central America In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 96. South & Central America In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 97. South & Central America In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 98. Brazil In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 99. Brazil In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 100. Brazil In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 101. Brazil In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 102. Argentina In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 103. Argentina In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 104. Argentina In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 105. Argentina In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 106. Rest of South & Central America In-Vitro Diagnostics Market, by Product and Services – Revenue and Forecast to 2030 (US$ Million)
Table 107. Rest of South & Central America In-Vitro Diagnostics Market, by Technology – Revenue and Forecast to 2030 (US$ Million)
Table 108. Rest of South & Central America In-Vitro Diagnostics Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 109. Rest of South & Central America In-Vitro Diagnostics Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 110. Organic Developments Done by Companies
Table 111. Inorganic Developments Done by Companies
Table 112. Glossary of Terms, In-Vitro Diagnostics Market
List of Figures
Figure 1. In-Vitro Diagnostics Market Segmentation, By Geography
Figure 2. PEST Analysis
Figure 3. In-Vitro Diagnostics Market: Key Industry Dynamics
Figure 4. In-Vitro Diagnostics Market: Impact Analysis of Drivers and Restraints
Figure 5. In-Vitro Diagnostics Market Revenue (US$ Mn), 2020 – 2030
Figure 6. Global In-Vitro Diagnostics Market, By Geography Forecast Analysis, 2022 & 2030
Figure 7. In-Vitro Diagnostics Market Revenue Share, by Product and Services 2022 & 2030 (%)
Figure 8. Reagents and Kits: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 9. Software and Services: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 10. Instruments: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 11. In-Vitro Diagnostics Market Revenue Share, by Technology 2022 & 2030 (%)
Figure 12. Immunoassay/ Immunochemistry: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 13. Clinical Chemistry: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 14. Molecular Diagnostics: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 15. Microbiology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 16. Blood Glucose Self-Monitoring: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 17. Coagulation and Hemostasis: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 18. Hematology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 19. Urinalysis: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 20. Others: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 21. In-Vitro Diagnostics Market Revenue Share, by Application 2022 & 2030 (%)
Figure 22. Infectious Diseases: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 23. Diabetes: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 24. Oncology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 25. Cardiology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 26. Autoimmune Diseases: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 27. Nephrology: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 28. Others: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 29. In-Vitro Diagnostics Market Revenue Share, by End User 2022 & 2030 (%)
Figure 30. Hospitals: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 31. Laboratories: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 32. Homecare: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 33. Others: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 34. North America: In-Vitro Diagnostics Market, by Key Country – Revenue (2022) (US$ Million)
Figure 35. North America In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 36. North America: In-Vitro Diagnostics Market, by Country, 2022 & 2030 (%)
Figure 37. US: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 38. Canada: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 39. Mexico: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 40. Europe: In-Vitro Diagnostics Market, by Key Country – Revenue (2022) (US$ Million)
Figure 41. Europe In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 42. Europe: In-Vitro Diagnostics Market, by Country, 2022 & 2030 (%)
Figure 43. Germany: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 44. France: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 45. UK: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 46. Italy: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 47. Spain: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 48. Rest of Europe: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 49. Asia Pacific: In-Vitro Diagnostics Market, by Key Country – Revenue (2022) (US$ Million)
Figure 50. Asia Pacific In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 51. Asia Pacific: In-Vitro Diagnostics Market, by Country, 2022 & 2030 (%)
Figure 52. China: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 53. Japan: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 54. India: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 55. Australia: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 56. South Korea: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 57. Rest of Asia Pacific: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 58. Middle East & Africa: In-Vitro Diagnostics Market, by Key Country – Revenue (2022) (US$ Million)
Figure 59. Middle East & Africa In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 60. Middle East & Africa: In-Vitro Diagnostics Market, by Country, 2022 & 2030 (%)
Figure 61. South Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 62. Saudi Arabia: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 63. UAE: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 64. Rest of Middle East & Africa: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 65. South & Central America: In-Vitro Diagnostics Market, By Key Country – Revenue (2022) (US$ Million)
Figure 66. South & Central America In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 67. South & Central America: In-Vitro Diagnostics Market, by Country, 2022 & 2030 (%)
Figure 68. Brazil: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 69. Argentina: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 70. Rest of South & Central America: In-Vitro Diagnostics Market – Revenue and Forecast to 2030 (US$ Million)
Figure 71. Growth Strategies in North America In-Vitro Diagnostics Market
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely - analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You'll receive access to the report within 4-6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we'll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we're happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you're considering, and we'll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report's scope, methodology, customization options, or which license suits you best, we're here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we'll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we'll ensure the issue is resolved quickly so you can access your report without interruption.
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.

Sep 2023
MRI-guided Focused Ultrasound Therapy Market
Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Application (Breast Cancer, Prostate Cancer, Liver Cancer, Pancreatic Cancer, Breast Lifting and Aesthetic Application, Nipple and Areola Preservation, Post Surgical Applications, and Others), End User (Healthcare Facilities, Diagnostic Imaging Centers, and Research Centers), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)

Sep 2023
Gene Therapy CDMO Market
Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type (Drug Development and Manufacturing, Testing and Regulatory Services, and Other Service Types), End User (Pharmaceutical Companies, Biopharmaceutical Companies, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)

Sep 2023
RT-PCR Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Reagents & Consumables, Instruments, and Software & Services), Application (Research Application, Clinical Application, and Forensic Application), End user (Hospitals and Diagnostic Centers, Pharmaceutical and Biotechnology Companies, Research Laboratories and Academic Institutes, Forensic Laboratories, and Clinical Research Organizations)

Sep 2023
dPCR Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Reagents & Consumables, Instruments, and Software & Services), Application (Research Application, Clinical Application, and Forensic Application), End user (Hospitals and Diagnostic Centers, Pharmaceutical and Biotechnology Companies, Research Laboratories and Academic Institutes, Forensic Laboratories, and Clinical Research Organizations)

Sep 2023
Oscillometry Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Device and Accessories), Technology (Impulse Oscillometry, Forced Oscillation Technique, and Hybrid Oscillometry Devices), Application (Asthma, COPD, and Others), End User (Hospitals, Diagnostic Laboratories, and Others)

Sep 2023
Prenatal Testing Services Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Diagnostic Type (Noninvasive and Invasive), Disease (Aneuploidy, Microdeletions, Structural Chromosomal Abnormalities, and Others), End User (Hospitals, Diagnostic Laboratories, Specialty Clinics, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)

Sep 2023
Joint Resurfacing Devices Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Knee, Hip, Shoulder, Ankle, and Others), Material (Metal, Ceramic, and Others), End User (Hospitals, Orthopedic Clinics, Ambulatory Surgical Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South and Central America)

Sep 2023
Embolization Plugs Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Application (Neurology, Peripheral Vascular Disease, Oncology, Urology, and Others), End User (Hospital, Ambulatory Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)


 Get Free Sample For
Get Free Sample For